Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts
- PMID: 18671756
- PMCID: PMC4498947
- DOI: 10.1111/j.1582-4934.2008.00448.x
Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts
Abstract
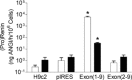
One important goal in cardiology is to prevent necrotic cell death in the heart. Necrotic cell death attracts neutrophils and monocytes into the injured myocardium. The consequences are fibrosis, remodelling and cardiac failure. The renin-angiotensin system promotes the development of cardiac failure. Recently, alternative renin transcripts have been identified lacking the signal sequence for a cotranslational transport to the endoplasmatic reticulum. These transcripts encode for a cytosolic renin with unknown functions. The expression of this alternative transcript increases after myocardial infarction. We hypothesized that cytosolic renin plays a role in survival and death of cardiomyocytes. To test this hypothesis, we overexpressed secretory or cytosolic renin in H9c2 cardiomyblasts and determined the rate of proliferation, necrosis and apoptosis. Proliferation rate, as indicated by BrdU incorporation into DNA, was reduced by secretory and cytosolic renin (cells transfected with control vector: 0.33 +/- 0.06; secretory renin: 0.12 +/- 0.02; P < 0.05; cytosolic renin: 0.15 +/- 0.03; P < 0.05). Necrosis was increased by secretory renin but decreased by cytosolic renin (LDH release after 10 days from cells transfected with control vector: 68.5 +/- 14.9; secretory renin: 100.0 +/- 0; cytosolic renin: 25.5 +/- 5.3% of content, each P < 0.05). Mitochondrial apoptosis, as indicated by phosphatidylserin translocation to the outer membrane, was unaffected by secretory renin but increased by cytosolic renin (controls: 23.8 +/- 3.9%; secretory renin: 22.1 +/- 4.7%; cytoplasmatic renin: 41.2 +/- 3.8%; P < 0.05). The data demonstrate that a cytosolic renin exists in cardiomyocytes, which in contradiction to secretory renin protects from necrosis but increases apoptosis. Non-secretory cytosolic renin can be considered as a new target for cardiac failure.
Figures






References
-
- Sadoshima J. Cytokine actions of angiotensin II. Circ Res. 2000;86:1187–9. - PubMed
-
- Pierzchalski P, Reiss K, Cheng W, et al. p53 induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp Cell Res. 1997;234:57–65. - PubMed
-
- Fortuno MA, Ravassa S, Etayo JC, et al. Overexpression of Bax protein and enhanced apoptosis in the left ventricle of spontaneously hypertensive rats: effects of AT1 blockade with losartan. Hypertension. 1998;32:280–6. - PubMed
-
- Cigola E, Kajstura J, Li B, et al. Angiotensin II activates programmed myocyte cell death in vitro. Exp Cell Res. 1997;231:363–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

