Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1
- PMID: 18671867
- PMCID: PMC2515828
- DOI: 10.1186/1744-8069-4-30
Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1
Abstract
Background: A number of prostaglandins (PGs) sensitize dorsal root ganglion (DRG) neurons and contribute to inflammatory hyperalgesia by signaling through specific G protein-coupled receptors (GPCRs). One mechanism whereby PGs sensitize these neurons is through modulation of "thermoTRPs," a subset of ion channels activated by temperature belonging to the Transient Receptor Potential ion channel superfamily. Acrid, electrophilic chemicals including cinnamaldehyde (CA) and allyl isothiocyanate (AITC), derivatives of cinnamon and mustard oil respectively, activate thermoTRP member TRPA1 via direct modification of channel cysteine residues.
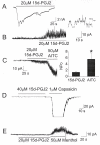
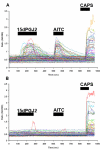
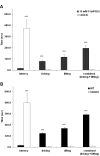
Results: Our search for endogenous chemical activators utilizing a bioactive lipid library screen identified a cyclopentane PGD2 metabolite, 15-deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2), as a TRPA1 agonist. Similar to CA and AITC, this electrophilic molecule is known to modify cysteines of cellular target proteins. Electophysiological recordings verified that 15d-PGJ2 specifically activates TRPA1 and not TRPV1 or TRPM8 (thermoTRPs also enriched in DRG). Accordingly, we identified a population of mouse DRG neurons responsive to 15d-PGJ2 and AITC that is absent in cultures derived from TRPA1 knockout mice. The irritant molecules that activate TRPA1 evoke nociceptive responses. However, 15d-PGJ2 has not been correlated with painful sensations; rather, it is considered to mediate anti-inflammatory processes via binding to the nuclear peroxisome proliferator-activated receptor gamma (PPARgamma). Our in vivo studies revealed that 15d-PGJ2 induced acute nociceptive responses when administered cutaneously. Moreover, mice deficient in the TRPA1 channel failed to exhibit such behaviors.
Conclusion: In conclusion, we show that 15d-PGJ2 induces acute nociception when administered cutaneously and does so via a TRPA1-specific mechanism.
Figures


References
-
- Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006. - PubMed
-
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–29. doi: 10.1016/S0092-8674(03)00158-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

