Hydrotropic polymer micelles containing acrylic acid moieties for oral delivery of paclitaxel
- PMID: 18672013
- PMCID: PMC2695849
- DOI: 10.1016/j.jconrel.2008.07.004
Hydrotropic polymer micelles containing acrylic acid moieties for oral delivery of paclitaxel
Abstract
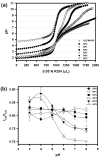
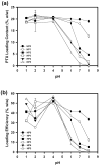
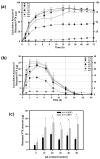
Hydrotropic polymers (HPs) and their micelles have been recently developed as vehicles for delivery of poorly water-soluble drugs, such as paclitaxel (PTX), by oral administration. The release of PTX from HP micelles, however, was slow and it took more than a day for complete release of the loaded PTX. Since the gastrointestinal (GI) transit time is known to be only several hours, pH-sensitive HP micelles were prepared for fast release of the loaded PTX responding to pH changes along the GI tract. Acrylic acid (AA) was introduced, as a release modulator, into HPs by copolymerization with 4-(2-vinylbenzyloxy)-N,N-(diethylnicotinamide) (VBODENA). The AA content was varied from 0% to 50% (in the molar ratio to VBODENA). HPs spontaneously produced micelles in water, and their critical micelle concentrations (CMCs) ranged from 31 microg/mL to 86 microg/mL. Fluorescence probe study using pyrene showed that blank HP micelles possessed a good pH sensitivity, which was clearly observed at relatively high AA contents and pH>6. The pH sensitivity also affected the PTX loading property. Above pH 5, the PTX loading content and loading efficiency in HP micelles were significantly reduced. Although this may be primarily due to the AA moieties, other factors may include PTX degradation and polymer aggregation. The PTX release from HP micelles with more than 20% (mol) AA contents was completed within 12 h in a simulated intestinal fluid (SIF, pH=6.5). The HP micelles without any AA moiety showed very slow release profiles. In the simulated gastric fluid (SGF, pH=1.6), severe degradation of the released PTX was observed. The pH-dependent release of PTX from HP micelles can be used to increase the bioavailability of PTX upon oral delivery.
Figures

Similar articles
-
Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles.J Control Release. 2004 Jun 18;97(2):249-57. doi: 10.1016/j.jconrel.2004.03.013. J Control Release. 2004. PMID: 15196752
-
Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization.Biomacromolecules. 2007 Jan;8(1):202-8. doi: 10.1021/bm060307b. Biomacromolecules. 2007. PMID: 17206808 Free PMC article.
-
Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery.J Control Release. 2005 Mar 21;103(2):341-53. doi: 10.1016/j.jconrel.2004.12.009. J Control Release. 2005. PMID: 15763618
-
Pharmacokinetics and biodistribution of paclitaxel-loaded pluronic P105 polymeric micelles.Arch Pharm Res. 2008 Apr;31(4):530-8. doi: 10.1007/s12272-001-1189-2. Epub 2008 May 1. Arch Pharm Res. 2008. PMID: 18449513
-
Formulation of drugs in block copolymer micelles: drug loading and release.Curr Pharm Des. 2006;12(36):4685-701. doi: 10.2174/138161206779026263. Curr Pharm Des. 2006. PMID: 17168772 Review.
Cited by
-
Quantitative structure-property relationship (QSPR) modeling of drug-loaded polymeric micelles via genetic function approximation.PLoS One. 2015 Mar 17;10(3):e0119575. doi: 10.1371/journal.pone.0119575. eCollection 2015. PLoS One. 2015. PMID: 25780923 Free PMC article.
-
Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs.Pharm Res. 2016 Jan;33(1):18-39. doi: 10.1007/s11095-015-1784-1. Epub 2015 Sep 17. Pharm Res. 2016. PMID: 26381278 Review.
-
Layer-by-layer nanoencapsulation of camptothecin with improved activity.Int J Pharm. 2014 Apr 25;465(1-2):218-27. doi: 10.1016/j.ijpharm.2014.01.041. Epub 2014 Feb 6. Int J Pharm. 2014. PMID: 24508806 Free PMC article.
-
Enhanced Oral Absorption of Icaritin by Using Mixed Polymeric Micelles Prepared with a Creative Acid-Base Shift Method.Molecules. 2021 Jun 6;26(11):3450. doi: 10.3390/molecules26113450. Molecules. 2021. PMID: 34204150 Free PMC article.
-
Self-assembled Tat nanofibers as effective drug carrier and transporter.ACS Nano. 2013 Jul 23;7(7):5965-77. doi: 10.1021/nn401667z. Epub 2013 Jun 18. ACS Nano. 2013. PMID: 23758167 Free PMC article.
References
-
- Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109(13):169–188. - PubMed
-
- Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101(13):59–68. - PubMed
-
- Coffman RE, Kildsig DO. Hydrotropic solubilization - Mechanistic studies. Pharm Res. 1996;13(10):1460–1463. - PubMed
-
- Holmberg K, Shah DO, Schwuger MJ Knovel (Firm) Handbook of applied surface and colloid chemistry. Wiley; Chichester, England ; New York: 2002.
-
- Mansur CRE, Spinelli LS, Lucas EF, Gonzalez G. The influence of a hydrotropic agent in the properties of aqueous solutions containing poly(ethylene oxide)-poly (propylene oxide) surfactants. Colloid Surface A. 1999;149(13):291–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

