The synaptic impact of the host immune response in a parkinsonian allograft rat model: Influence on graft-derived aberrant behaviors
- PMID: 18672063
- PMCID: PMC2886670
- DOI: 10.1016/j.nbd.2008.06.018
The synaptic impact of the host immune response in a parkinsonian allograft rat model: Influence on graft-derived aberrant behaviors
Abstract
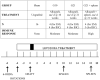
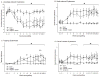
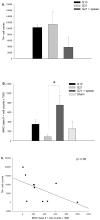
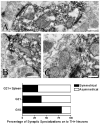
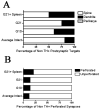
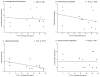
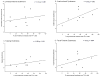
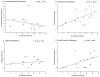
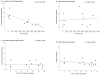
Graft-induced dyskinesias (GIDs), side-effects found in clinical grafting trials for Parkinson's disease (PD), may be associated with the withdrawal of immunosuppression. The goal of this study was to determine the role of the immune response in GIDs. We examined levodopa-induced dyskinesias (LIDs), GID-like behaviors, and synaptic ultrastructure in levodopa-treated, grafted, parkinsonian rats with mild (sham), moderate (allografts) or high (allografts plus peripheral spleen cell injections) immune activation. Grafts attenuated amphetamine-induced rotations and LIDs, but two abnormal motor syndromes (tapping stereotypy, litter retrieval/chewing) emerged and increased with escalating immune activation. Immunohistochemical analyses confirmed immune activation and graft survival. Ultrastructural analyses showed increases in tyrosine hydroxylase-positive (TH+) axo-dendritic synapses, TH+ asymmetric specializations, and non-TH+ perforated synapses in grafted, compared to intact, striata. These features were exacerbated in rats with the highest immune activation and correlated statistically with GID-like behaviors, suggesting that immune-mediated aberrant synaptology may contribute to graft-induced aberrant behaviors.
Figures



References
-
- Anglade P, Mouatt-Prigent A, Agid Y, Hirsch E. Synaptic plasticity in the caudate nucleus of patients with Parkinson’s disease. Neurodegeneration. 1996;5:121–8. - PubMed
-
- Arbuthnott G, Dunnett S, MacLeod N. Electrophysiological properties of single units in dopamine-rich mesencephalic transplants in rat brain. Neurosci Lett. 1985;57:205–10. - PubMed
-
- Bains JS, Oliet SH. Glia: they make your memories stick! Trends Neurosci. 2007;30:417–24. - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNF alpha. Science. 2002;295:2282–5. - PubMed
-
- Bolam JP. Synapses of identified neurons in the neostriatum. Ciba Found Symp. 1984;107:30–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

