Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies
- PMID: 18673209
- PMCID: PMC2562687
- DOI: 10.2174/187152708784936608
Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies
Abstract
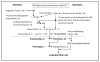
Today there exists only one FDA-approved treatment for ischemic stroke; i.e., the serine protease tissue-type plasminogen activator (tPA). In the aftermath of the failed stroke clinical trials with the nitrone spin trap/radical scavenger, NXY-059, a number of articles raised the question: are we doing the right thing? Is the animal research truly translational in identifying new agents for stroke treatment? This review summarizes the current state of affairs with plasminogen activators in thrombolytic therapy. In addition to therapeutic value, potential side effects of tPA also exist that aggravate stroke injury and offset the benefits provided by reperfusion of the occluded artery. Thus, combinational options (ultrasound alone or with microspheres/nanobubbles, mechanical dissociation of clot, activated protein C (APC), plasminogen activator inhibitor-1 (PAI-1), neuroserpin and CDP-choline) that could offset tPA toxic side effects and improve efficacy are also discussed here. Desmoteplase, a plasminogen activator derived from the saliva of Desmodus rotundus vampire bat, antagonizes vascular tPA-induced neurotoxicity by competitively binding to low-density lipoprotein related-receptors (LPR) at the blood-brain barrier (BBB) interface, minimizing the tPA uptake into brain parenchyma. tPA can also activate matrix metalloproteinases (MMPs), a family of endopeptidases comprised of 24 mammalian enzymes that primarily catalyze the turnover and degradation of the extracellular matrix (ECM). MMPs have been implicated in BBB breakdown and neuronal injury in the early times after stroke, but also contribute to vascular remodeling, angiogenesis, neurogenesis and axonal regeneration during the later repair phase after stroke. tPA, directly or by activation of MMP-9, could have beneficial effects on recovery after stroke by promoting neurovascular repair through vascular endothelial growth factor (VEGF). However, any treatment regimen directed at MMPs must consider their pleiotropic nature and the likelihood of either beneficial or detrimental effects that might depend on the timing of the treatment in relation to the stage of brain injury.
Conflict of interest statement
Figures


References
-
- Lo EH, Broderick JP, Moskowitz MA. Stroke. 2004;35:354–356. - PubMed
-
- Young AR, Ali C, Duretete A, Vivien D. J Neurochem. 2007;103:1302–1309. - PubMed
-
- Adibhatla RM, Hatcher JF. Free Radic Biol Med. 2006;40:376–387. - PubMed
-
- Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao F. J Biol Chem. 2006;281:6718–6725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
