Lipid content of brain, brain membrane lipid domains, and neurons from acid sphingomyelinase deficient mice
- PMID: 18673449
- PMCID: PMC2581651
- DOI: 10.1111/j.1471-4159.2008.05591.x
Lipid content of brain, brain membrane lipid domains, and neurons from acid sphingomyelinase deficient mice
Abstract
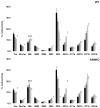
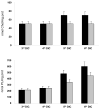
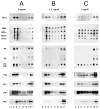
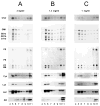
The cholesterol, sphingolipid, and glycerophospholipid content of total brain, of detergent-resistant membranes prepared from the total brain, and of cerebellar granule cells differentiated in culture from wild type (WT) and acid sphingomyelinase knockout (ASMKO) were studied. Brains derived from 7-month-old ASMKO animals showed a fivefold higher level of sphingomyelin and a significant increase in ganglioside content, mainly because of monosialogangliosides GM3 and GM2 accumulation, while the cholesterol and glycerophospholipid content was unchanged with respect to WT animals. An increase in sphingomyelin, but not in gangliosides, was also detected in cultured cerebellar granule neurons from ASMKO mice, indicating that ganglioside accumulation is not a direct consequence of the enzyme defect. When a detergent-resistant membrane fraction was prepared from ASMKO brains, we observed that a higher detergent-to-protein ratio was needed than in WT animals. This likely reflects a reduced fluidity in restricted membrane areas because of a higher enrichment in sphingolipids in the case of ASMKO brain.
Figures

References
-
- Andres R, Cabezas JA, Llanillo M. Changes in forebrain, cerebellum and brain stem phospholipid patterns from mothers and newborn rats after chronic pentazocine treatment during the gestation period. Ital J Biochem. 1988;37:275–283. - PubMed
-
- Baron W, Decker L, Colognato H, Ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. - PubMed
-
- Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

