Testing for nested oscillation
- PMID: 18674562
- PMCID: PMC2675174
- DOI: 10.1016/j.jneumeth.2008.06.035
Testing for nested oscillation
Abstract
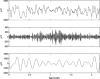
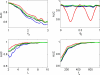
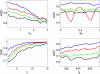
Nested oscillation occurs when the amplitude of a faster rhythm is coupled to the phase of a slower rhythm. It has been proposed to underlie the discrete nature of perception and the capacity of working memory and is a phenomenon observable in human brain imaging data. This paper compares three published methods for detecting nested oscillation and a fourth method proposed in this paper. These are: (i) the modulation index, (ii) the phase-locking value (PLV), (iii) the envelope-to-signal correlation (ESC) and (iv) a general linear model (GLM) measure derived from ESC. We applied the methods to electrocorticographic (ECoG) data recorded during a working-memory task and to data from a simulated hippocampal interneuron network. Further simulations were then made to address the dependence of each measure on signal to noise level, coupling phase, epoch length, sample rate, signal nonstationarity, and multi-phasic coupling. Our overall conclusion is that the GLM measure is the best all-round approach for detecting nested oscillation.
Figures








References
-
- Bruns A., Eckhorn R. Task-related coupling from high- to low-frequency signals among visual cortical areas in human subdural recordings. Int J Psychophysiol. 2004;51:97–116. - PubMed
-
- Bretthorst L. Springer-Verlag; New York: 1988. Bayesian spectrum analysis and parameter estimation. Lecture notes in statistics.
-
- Buzsaki G. Oxford University Press; New York: 2006. Rhythms of the brain.
-
- Chen C., Kiebel S., Friston K. Dynamic causal modelling of induced responses. Neuroimage. 2008;41(4):1293–1312. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

