Decreased glutamate transport enhances excitability in a rat model of cortical dysplasia
- PMID: 18674619
- PMCID: PMC2643870
- DOI: 10.1016/j.nbd.2008.07.003
Decreased glutamate transport enhances excitability in a rat model of cortical dysplasia
Abstract
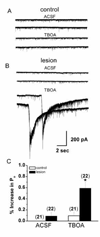
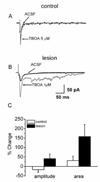
Glutamate transporters function to maintain low levels of extracellular glutamate and play an important role in synaptic transmission at many synapses. Disruption of glutamate transporter function or expression can result in increased extracellular glutamate levels. Alterations in glutamate transporter expression have been reported in human epilepsy and animal seizure models. Functional electrophysiological changes that occur when transporter expression is disrupted in chronic epilepsy models have not been examined. Here, we used a freeze-induced model of cortical dysplasia to test the role of glutamate transporters in synaptic hyperexcitability. We report that inhibiting glutamate transporters with the non-selective antagonist, DL-threo-beta-benzylozyaspartic acid (TBOA) preferentially prolongs postsynaptic currents (PSCs) and decreases the threshold for evoking epileptiform activity in lesioned compared to control cortex. The effect of inhibiting uptake is mediated primarily by the glia glutamate transporter (GLT-1) since the selective antagonist dihydrokainate (DHK) mimicked the effects of TBOA. The effect of uptake inhibition is mediated by activation of N-methyl-D-aspartate (NMDA) receptors since D-(-)-2-amino-5-phosphonovaleric acid (APV) prevents TBOA-induced effects. Neurons in lesioned cortex also have a larger tonic NMDA current. These results indicate that chronic changes in glutamate transporters and NMDA receptors contribute to hyperexcitability in cortical dysplasia.
Figures


Similar articles
-
Glutamate transporters regulate excitability in local networks in rat neocortex.Neuroscience. 2004;127(3):625-35. doi: 10.1016/j.neuroscience.2004.05.030. Neuroscience. 2004. PMID: 15283962
-
Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn.J Neurophysiol. 2009 Apr;101(4):2041-51. doi: 10.1152/jn.91138.2008. Epub 2009 Feb 11. J Neurophysiol. 2009. PMID: 19211657 Free PMC article.
-
Ncm-D-aspartate: a novel caged D-aspartate suitable for activation of glutamate transporters and N-methyl-D-aspartate (NMDA) receptors in brain tissue.Neuropharmacology. 2005 Nov;49(6):831-42. doi: 10.1016/j.neuropharm.2005.07.018. Epub 2005 Sep 15. Neuropharmacology. 2005. PMID: 16169022
-
Modification of epileptiform discharges in neocortical neurons following glutamate uptake inhibition.Epilepsia. 2005;46 Suppl 5:129-33. doi: 10.1111/j.1528-1167.2005.01020.x. Epilepsia. 2005. PMID: 15987267
-
Glutamate transport blockade has a differential effect on AMPA and NMDA receptor-mediated synaptic transmission in the developing barrel cortex.Neuropharmacology. 2000 Mar 3;39(5):725-32. doi: 10.1016/s0028-3908(99)00270-1. Neuropharmacology. 2000. PMID: 10699439
Cited by
-
Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia.J Neurophysiol. 2011 Nov;106(5):2189-200. doi: 10.1152/jn.00164.2011. Epub 2011 Jul 27. J Neurophysiol. 2011. PMID: 21795624 Free PMC article.
-
Role of astrocytes in epilepsy.Cold Spring Harb Perspect Med. 2015 Mar 2;5(3):a022434. doi: 10.1101/cshperspect.a022434. Cold Spring Harb Perspect Med. 2015. PMID: 25732035 Free PMC article. Review.
-
Astrocyte membrane properties are altered in a rat model of developmental cortical malformation but single-cell astrocytic glutamate uptake is robust.Neurobiol Dis. 2016 May;89:157-68. doi: 10.1016/j.nbd.2016.02.012. Epub 2016 Feb 10. Neurobiol Dis. 2016. PMID: 26875663 Free PMC article.
-
Soluble Aβ oligomers impair hippocampal LTP by disrupting glutamatergic/GABAergic balance.Neurobiol Dis. 2016 Jan;85:111-121. doi: 10.1016/j.nbd.2015.10.019. Epub 2015 Oct 22. Neurobiol Dis. 2016. PMID: 26525100 Free PMC article.
-
α2δ-1 Signaling Drives Cell Death, Synaptogenesis, Circuit Reorganization, and Gabapentin-Mediated Neuroprotection in a Model of Insult-Induced Cortical Malformation.eNeuro. 2017 Nov 6;4(5):ENEURO.0316-17.2017. doi: 10.1523/ENEURO.0316-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29109971 Free PMC article.
References
-
- Aamodt S, Constantine-Paton M. The role of neuronal activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. - PubMed
-
- Andre VM, Flores-Hernandez J, Cepeda C, Starling AJ, Nguyen S, Lobo MK, Vinters HV, Levine MS, Mathern GW. NMDA receptor alterations in neurons from pediatric cortical dysplasia tissue. Cereb Cortex. 2004;14:634–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

