DNA packaging motor assembly intermediate of bacteriophage phi29
- PMID: 18674782
- PMCID: PMC2614240
- DOI: 10.1016/j.jmb.2008.04.034
DNA packaging motor assembly intermediate of bacteriophage phi29
Abstract
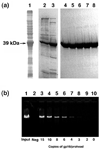
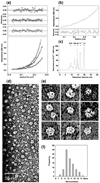
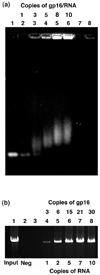
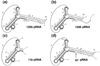
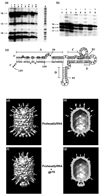
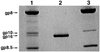
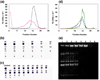
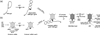
Unraveling the structure and assembly of the DNA packaging ATPases of the tailed double-stranded DNA bacteriophages is integral to understanding the mechanism of DNA translocation. Here, the bacteriophage phi29 packaging ATPase gene product 16 (gp16) was overexpressed in soluble form in Bacillus subtilis (pSAC), purified to near homogeneity, and assembled to the phi29 precursor capsid (prohead) to produce a packaging motor intermediate that was fully active in in vitro DNA packaging. The formation of higher oligomers of the gp16 from monomers was concentration dependent and was characterized by analytical ultracentrifugation, gel filtration, and electron microscopy. The binding of multiple copies of gp16 to the prohead was dependent on the presence of an oligomer of 174- or 120-base prohead RNA (pRNA) fixed to the head-tail connector at the unique portal vertex of the prohead. The use of mutant pRNAs demonstrated that gp16 bound specifically to the A-helix of pRNA, and ribonuclease footprinting of gp16 on pRNA showed that gp16 protected the CC residues of the CCA bulge (residues 18-20) of the A-helix. The binding of gp16 to the prohead/pRNA to constitute the complete and active packaging motor was confirmed by cryo-electron microscopy three-dimensional reconstruction of the prohead/pRNA/gp16 complex. The complex was capable of supercoiling DNA-gp3 as observed previously for gp16 alone; therefore, the binding of gp16 to the prohead, rather than first to DNA-gp3, represents an alternative packaging motor assembly pathway.
Figures


References
-
- Catalano CE. Viral genome packaging machines: an overview. In: Catalano CE, editor. Viral genome packaging machines: Genetics, structure and mechanism. Georgetown, TX: 2005. pp. 1–4. Landes Bioscience/Eurekah.com.
-
- Jardine PJ, Anderson DL. DNA packaging in double-stranded DNA phages. In: Calendar R, editor. The bacteriophages. New York: oxford press; 2006. pp. 49–65.
-
- Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C. The bacteriophage phi29 portal motor can package DNA against a large internal force. Nature (London) 2001;413:748–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

