D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila
- PMID: 18674913
- PMCID: PMC2603029
- DOI: 10.1016/j.cub.2008.07.028
D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila
Abstract
Background: Extended wakefulness disrupts acquisition of short-term memories in mammals. However, the underlying molecular mechanisms triggered by extended waking and restored by sleep are unknown. Moreover, the neuronal circuits that depend on sleep for optimal learning remain unidentified.
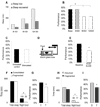
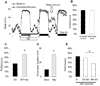
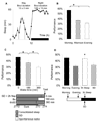
Results: Learning was evaluated with aversive phototaxic suppression. In this task, flies learn to avoid light that is paired with an aversive stimulus (quinine-humidity). We demonstrate extensive homology in sleep-deprivation-induced learning impairment between flies and humans. Both 6 hr and 12 hr of sleep deprivation are sufficient to impair learning in Canton-S (Cs) flies. Moreover, learning is impaired at the end of the normal waking day in direct correlation with time spent awake. Mechanistic studies indicate that this task requires intact mushroom bodies (MBs) and requires the dopamine D1-like receptor (dDA1). Importantly, sleep-deprivation-induced learning impairments could be rescued by targeted gene expression of the dDA1 receptor to the MBs.
Conclusions: These data provide direct evidence that extended wakefulness disrupts learning in Drosophila. These results demonstrate that it is possible to prevent the effects of sleep deprivation by targeting a single neuronal structure and identify cellular and molecular targets adversely affected by extended waking in a genetically tractable model organism.
Figures



Similar articles
-
D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila.J Neurosci. 2007 Jul 18;27(29):7640-7. doi: 10.1523/JNEUROSCI.1167-07.2007. J Neurosci. 2007. PMID: 17634358 Free PMC article.
-
Notch signaling modulates sleep homeostasis and learning after sleep deprivation in Drosophila.Curr Biol. 2011 May 24;21(10):835-40. doi: 10.1016/j.cub.2011.04.001. Epub 2011 May 5. Curr Biol. 2011. PMID: 21549599 Free PMC article.
-
Persistent short-term memory defects following sleep deprivation in a drosophila model of Parkinson disease.Sleep. 2009 Aug;32(8):984-92. doi: 10.1093/sleep/32.8.984. Sleep. 2009. PMID: 19725249 Free PMC article.
-
Olfactory learning in Drosophila.Physiology (Bethesda). 2010 Dec;25(6):338-46. doi: 10.1152/physiol.00026.2010. Physiology (Bethesda). 2010. PMID: 21186278 Free PMC article. Review.
-
Toward nanoscale localization of memory engrams in Drosophila.J Neurogenet. 2020 Mar;34(1):151-155. doi: 10.1080/01677063.2020.1715973. Epub 2020 Jan 27. J Neurogenet. 2020. PMID: 31985306 Review.
Cited by
-
Behavioral consequences of dopamine deficiency in the Drosophila central nervous system.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):834-9. doi: 10.1073/pnas.1010930108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187381 Free PMC article.
-
Aging and circadian dysfunction increase alcohol sensitivity and exacerbate mortality in Drosophila melanogaster.Exp Gerontol. 2017 Oct 15;97:49-59. doi: 10.1016/j.exger.2017.07.014. Epub 2017 Jul 25. Exp Gerontol. 2017. PMID: 28750752 Free PMC article.
-
Identification of Genes that Maintain Behavioral and Structural Plasticity during Sleep Loss.Front Neural Circuits. 2017 Oct 23;11:79. doi: 10.3389/fncir.2017.00079. eCollection 2017. Front Neural Circuits. 2017. PMID: 29109678 Free PMC article.
-
A neural circuit mechanism integrating motivational state with memory expression in Drosophila.Cell. 2009 Oct 16;139(2):416-27. doi: 10.1016/j.cell.2009.08.035. Cell. 2009. PMID: 19837040 Free PMC article.
-
Short-Term Memory Deficits in the SLEEP Inbred Panel.Clocks Sleep. 2019 Dec;1(4):471-488. doi: 10.3390/clockssleep1040036. Epub 2019 Oct 28. Clocks Sleep. 2019. PMID: 32596662 Free PMC article.
References
-
- Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–s1067. - PubMed
-
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. - PubMed
-
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. - PubMed
-
- Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. - PubMed
-
- Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987;4:65–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

