Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model
- PMID: 18675385
- PMCID: PMC3014108
- DOI: 10.1016/j.bone.2008.06.019
Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model
Abstract
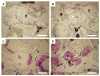
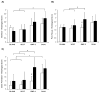
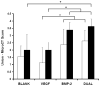
This study investigated the effects of dual delivery of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2 (BMP-2) for bone regeneration in a rat cranial critical size defect. Four groups of scaffolds were generated with VEGF (12 microg), BMP-2 (2 mug), both VEGF (12 microg) and BMP-2 (2 microg), or no growth factor released from gelatin microparticles incorporated within the scaffold pores. These scaffolds were implanted within an 8 mm rat cranial critical size defect (n=8-9 for each group). At 4 and 12 weeks, implants were retrieved and evaluated by microcomputed tomography (microCT) and histological scoring analysis. Additionally, 4 week animals were perfused with a radiopaque material to visualize and quantify blood vessel formation. Histological analysis revealed that for all groups at 4 weeks, a majority of the porous scaffold volume was filled with vascularized fibrous tissue; however, bone formation appeared most abundant in the dual release group at this time. At 12 weeks, both dual release and BMP-2 groups showed large amounts of bone formation within the scaffold pores and along the outer surfaces of the scaffold; osteoid secretion and mineralization were apparent, and new bone was often in close or direct contact with the scaffold interface. MicroCT results showed no significant difference among groups for blood vessel formation at 4 weeks (<4% blood vessel volume); however, the dual release group showed significantly higher bone formation (16.1+/-9.2% bone volume) than other groups at this time. At 12 weeks, dual release and BMP-2 groups exhibited significantly higher bone formation (39.7+/-14.1% and 37.4+/-18.8% bone volume, respectively) than either the VEGF group or blank scaffolds (6.3+/-4.8% and 7.8+/-7.1% bone volume, respectively). This work indicates a synergistic effect of the dual delivery of VEGF and BMP-2 on bone formation at 4 weeks and suggests an interplay between these growth factors for early bone regeneration. For the doses investigated, the results show that the addition of VEGF does not affect the amount of bone formation achieved by BMP-2 at 12 weeks; however, they also indicate that delivery of both growth factors may enhance bone bridging and union of the critical size defect compared to delivery of BMP-2 alone.
Figures




References
-
- Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Rosemont: American Academy of Orthopaedic Surgeons; 1999.
-
- Glowacki J. Angiogenesis in fracture repair. Clin Orthop. 1998:S82–9. - PubMed
-
- Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–9. - PubMed
-
- Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–8. - PubMed
-
- Schmid J, Wallkamm B, Hammerle CH, Gogolewski S, Lang NP. The significance of angiogenesis in guided bone regeneration. A case report of a rabbit experiment. Clin Oral Implants Res. 1997;8:244–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

