The MGTX experience: challenges in planning and executing an international, multicenter clinical trial
- PMID: 18675464
- PMCID: PMC2654214
- DOI: 10.1016/j.jneuroim.2008.05.031
The MGTX experience: challenges in planning and executing an international, multicenter clinical trial
Erratum in
- J Neuroimmunol. 2009 Dec 10;217(1-2):103
Abstract
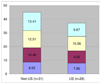
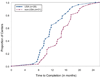
We present our experience planning and launching a multinational, NIH/NINDS funded study of thymectomy in myasthenia gravis. We highlight the additional steps required for international sites and analyze and contrast the time investment required to bring U.S. and non-U.S. sites into full regulatory compliance. Results show the mean time for non-U.S. centers to achieve regulatory approval was significantly longer (mean 13.4+/0.96 [corrected] months) than for U.S. sites (9.67+/0.74 [corrected] months; p=0.003, [corrected] t-test). The delay for non-U.S. sites was mainly attributable to Federalwide Assurance certification and State Department clearance.
Figures
References
-
- Burris S. Regulatory innovation in the governance of human subjects research: A cautionary tale and some modest proposals. Regulation & Governance. 2008;2:65–84.
-
- Chiu H-C, Vincent A, Nemsom-Davis J, Hsieh K-H, Hung T-p. Myasthenia gravis: population differences in disease expression and acetylcholine receptor antibody titers between Chinese and Caucasians. Neurology. 1987;37:1854–1857. - PubMed
-
- Collins JF, Garg R, Teo KK, Williford WO, Howell CL, on behalf of the DIG Investigators The role of the data coordinating center in the IRB review and approval process: the DIG trial experience. Controlled Clinical Trials. 2003;24:306S–315S. - PubMed
-
- Compston DAS, Vincent A, Newsom-Davis J, Batchelor JR. Clinical, pathological, HLA antigen, and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980;103:579–601. - PubMed
-
- Duley L, Antman K, Arena J, Avezum A, Blumenthal M, Bosch J, Chrolavicius S, Li T, Ounpuu S, Perez AC, Sleight P, Svard R, Temple R, Tsouderous Y, Yunis C, Yusuf S. Specific barriers to the conduct of randomized trials. Clin Trials. 2008;5:40–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical