Novel mRNA isoforms of the sodium channels Na(v)1.2, Na(v)1.3 and Na(v)1.7 encode predicted two-domain, truncated proteins
- PMID: 18675520
- PMCID: PMC2726981
- DOI: 10.1016/j.neuroscience.2008.04.060
Novel mRNA isoforms of the sodium channels Na(v)1.2, Na(v)1.3 and Na(v)1.7 encode predicted two-domain, truncated proteins
Abstract
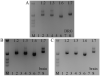
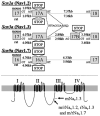
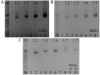
The expression of voltage-gated sodium channels is regulated at multiple levels, and in this study we addressed the potential for alternative splicing of the Na(v)1.2, Na(v)1.3, Na(v)1.6 and Na(v)1.7 mRNAs. We isolated novel mRNA isoforms of Na(v)1.2 and Na(v)1.3 from adult mouse and rat dorsal root ganglia (DRG), Na(v)1.3 and Na(v)1.7 from adult mouse brain, and Na(v)1.7 from neonatal rat brain. These alternatively spliced isoforms introduce an additional exon (Na(v)1.2 exon 17A and topologically equivalent Na(v)1.7 exon 16A) or exon pair (Na(v)1.3 exons 17A and 17B) that contain an in-frame stop codon and result in predicted two-domain, truncated proteins. The mouse and rat orthologous exon sequences are highly conserved (94-100% identities), as are the paralogous Na(v)1.2 and Na(v)1.3 exons (93% identity in mouse) to which the Na(v)1.7 exon has only 60% identity. Previously, Na(v)1.3 mRNA has been shown to be upregulated in rat DRG following peripheral nerve injury, unlike the downregulation of all other sodium channel transcripts. Here we show that the expression of Na(v)1.3 mRNA containing exons 17A and 17B is unchanged in mouse following peripheral nerve injury (axotomy), whereas total Na(v)1.3 mRNA expression is upregulated by 33% (P=0.003), suggesting differential regulation of the alternatively spliced transcripts. The alternatively spliced rodent exon sequences are highly conserved in both the human and chicken genomes, with 77-89% and 72-76% identities to mouse, respectively. The widespread conservation of these sequences strongly suggests an additional level of regulation in the expression of these channels, that is also tissue-specific.
Figures



Similar articles
-
Novel isoforms of the sodium channels Nav1.8 and Nav1.5 are produced by a conserved mechanism in mouse and rat.J Biol Chem. 2004 Jun 4;279(23):24826-33. doi: 10.1074/jbc.M401281200. Epub 2004 Mar 26. J Biol Chem. 2004. PMID: 15047701 Free PMC article.
-
A subtle alternative splicing event of the Na(V)1.8 voltage-gated sodium channel is conserved in human, rat, and mouse.J Mol Neurosci. 2010 Jun;41(2):310-4. doi: 10.1007/s12031-009-9315-3. Epub 2009 Dec 2. J Mol Neurosci. 2010. PMID: 19953341
-
Alternative splicing of the sodium channel SCN8A predicts a truncated two-domain protein in fetal brain and non-neuronal cells.J Biol Chem. 1997 Sep 19;272(38):24008-15. doi: 10.1074/jbc.272.38.24008. J Biol Chem. 1997. PMID: 9295353
-
Functional regulation of alternatively spliced Na+/Ca2+ exchanger (NCX1) isoforms.Ann N Y Acad Sci. 2002 Nov;976:187-96. doi: 10.1111/j.1749-6632.2002.tb04740.x. Ann N Y Acad Sci. 2002. PMID: 12502560 Review.
-
Structure of the sodium channel gene SCN11A: evidence for intron-to-exon conversion model and implications for gene evolution.Mol Neurobiol. 2002 Oct-Dec;26(2-3):235-50. doi: 10.1385/MN:26:2-3:235. Mol Neurobiol. 2002. PMID: 12428758 Review.
Cited by
-
Molecular characterization and functional expression of the DSC1 channel.Insect Biochem Mol Biol. 2011 Jul;41(7):451-8. doi: 10.1016/j.ibmb.2011.04.010. Epub 2011 May 7. Insect Biochem Mol Biol. 2011. PMID: 21571069 Free PMC article.
-
Rbfox proteins regulate alternative splicing of neuronal sodium channel SCN8A.Mol Cell Neurosci. 2012 Feb;49(2):120-6. doi: 10.1016/j.mcn.2011.10.005. Epub 2011 Oct 21. Mol Cell Neurosci. 2012. PMID: 22044765 Free PMC article.
-
The expression of ELK transcription factors in adult DRG: Novel isoforms, antisense transcripts and upregulation by nerve damage.Mol Cell Neurosci. 2010 Jun;44(2):165-77. doi: 10.1016/j.mcn.2010.03.005. Epub 2010 Mar 18. Mol Cell Neurosci. 2010. PMID: 20304071 Free PMC article.
-
Targeted disruption of the orphan receptor Gpr151 does not alter pain-related behaviour despite a strong induction in dorsal root ganglion expression in a model of neuropathic pain.Mol Cell Neurosci. 2017 Jan;78:35-40. doi: 10.1016/j.mcn.2016.11.010. Epub 2016 Nov 30. Mol Cell Neurosci. 2017. PMID: 27913310 Free PMC article.
-
Sodium channelopathies in neurodevelopmental disorders.Nat Rev Neurosci. 2021 Mar;22(3):152-166. doi: 10.1038/s41583-020-00418-4. Epub 2021 Feb 2. Nat Rev Neurosci. 2021. PMID: 33531663 Free PMC article. Review.
References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

