MHC class I assembly: out and about
- PMID: 18675588
- PMCID: PMC2774448
- DOI: 10.1016/j.it.2008.06.004
MHC class I assembly: out and about
Abstract
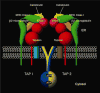
The assembly of major histocompatibility complex (MHC) class I molecules with peptides is orchestrated by several assembly factors including the transporter associated with antigen processing (TAP) and tapasin, the endoplasmic reticulum (ER) oxido-reductases ERp57 and protein disulfide isomerase (PDI), the lectin chaperones calnexin and calreticulin, and the ER aminopeptidase (ERAAP). Typically, MHC class I molecules present endogenous antigens to cytotoxic T lymphocytes (CTLs). However, the initiation of CD8(+) T-cell responses against many pathogens and tumors also requires the presentation of exogenous antigens by MHC class I molecules. We discuss recent developments relating to interactions and mechanisms of function of the various assembly factors and pathways by which exogenous antigens access MHC class I molecules.
Figures


References
-
- Cresswell P, et al. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. - PubMed
-
- Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. - PubMed
-
- Scholz C, Tampe R. The intracellular antigen transport machinery TAP in adaptive immunity and virus escape mechanisms. J Bioenerg Biomembr. 2005;37:509–515. - PubMed
-
- Koch J, et al. Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP) J Biol Chem. 2004;279:10142–10147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

