Eye formation in the absence of retina
- PMID: 18675797
- PMCID: PMC3104407
- DOI: 10.1016/j.ydbio.2008.07.009
Eye formation in the absence of retina
Abstract
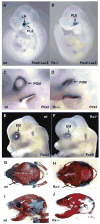
Eye development is a complex process that involves the formation of the retina and the lens, collectively called the eyeball, as well as the formation of auxiliary eye structures such as the eyelid, lacrimal gland, cornea and conjunctiva. The developmental requirements for the formation of each individual structure are only partially understood. We have shown previously that the homeobox-containing gene Rx is a key component in eye formation, as retinal structures do not develop and retina-specific gene expression is not observed in Rx-deficient mice. In addition, Rx-/- embryos do not develop any lens structure, despite the fact that Rx is not expressed in the lens. This demonstrates that during normal mammalian development, retina-specific gene expression is necessary for lens formation. In this paper we show that lens formation can be restored in Rx-deficient embryos experimentally, by the elimination of beta-catenin expression in the head surface ectoderm. This suggests that beta-catenin is involved in lens specification either through Wnt signaling or through its function in cell adhesion. In contrast to lens formation, we demonstrate that the development of auxiliary eye structures does not depend on retina-specific gene expression or retinal morphogenesis. These results point to the existence of two separate developmental processes involved in the formation of the eye and its associated structures. One involved in the formation of the eyeball and the second involved in the formation of the auxiliary eye structures.
Figures


References
-
- Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26:78–98. - PubMed
-
- Aota S, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev Biol. 2003;257:1–13. - PubMed
-
- Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers EH, Jamrich M. Regulation of vertebrate eye development by Rx genes. Int J Dev Biol. 2004;48:761–770. - PubMed
-
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

