Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)
- PMID: 18676075
- PMCID: PMC2897819
- DOI: 10.1053/j.ajkd.2008.05.027
Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)
Abstract
Background: Dyslipidemia is common in patients with chronic kidney disease. The role of statin therapy in the progression of kidney disease is unclear.
Study design: Prospective randomized clinical trial, post hoc analyses.
Setting & participants: 10,060 participants in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (lipid-lowering component) stratified by baseline estimated glomerular filtration rate (eGFR): less than 60, 60 to 89, and 90 or greater mL/min/1.73 m(2). Mean follow-up was 4.8 years.
Intervention: Randomized; pravastatin, 40 mg/d, or usual care.
Outcomes & measurements: Total, high-density lipoprotein, and low-density lipoprotein cholesterol; end-stage renal disease (ESRD), eGFR.
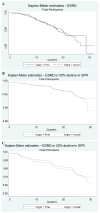
Results: Through year 6, total cholesterol levels decreased in the pravastatin (-20.7%) and usual-care groups (-11.2%). No significant differences were seen between groups for rates of ESRD (1.36 v 1.45/100 patient-years; P = 0.9), composite end points of ESRD and 50% or 25% decrease in eGFR, or rate of change in eGFR. Findings were consistent across eGFR strata. In patients with eGFR of 90 mL/min/1.73 m(2) or greater, the pravastatin arm tended to have a higher eGFR.
Limitations: Proteinuria data unavailable, post hoc analyses, unconfirmed validity of the Modification of Diet in Renal Disease Study equation in normal eGFR range, statin drop-in rate in usual-care group with small cholesterol differential between groups.
Conclusions: In hypertensive patients with moderate dyslipidemia and decreased eGFR, pravastatin was not superior to usual care in preventing clinical renal outcomes. This was consistent across the strata of baseline eGFR. However, benefit from statin therapy may depend on the degree of the cholesterol level decrease achieved.
Trial registration: ClinicalTrials.gov NCT00000542.
Figures





Comment in
-
Statins for slowing kidney disease progression: an as yet unproven indication.Am J Kidney Dis. 2008 Sep;52(3):391-4. doi: 10.1053/j.ajkd.2008.07.010. Am J Kidney Dis. 2008. PMID: 18725010 No abstract available.
References
-
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–266. - PubMed
-
- Kaysen GA. Dyslipidemia in chronic kidney disease: Causes and consequences. Kidney Int. 2006;70 (Supplement 104S):S55–S58.
-
- Manttari M, Tiula E, Alikoski T, Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995;26:670–675. - PubMed
-
- Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, Buring JE, Gaziano JM. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–2091. - PubMed
-
- Kosch M, Barenbrock M, Suwelack B, Schaefer RM, Rahn KH, Hausberg M. Effect of a 3-year therapy with the 3-hydroxy-3-methylglutaryl coenzyme a reductase-inhibitor fluvastatin on endothelial function and distensibility of large arteries in hypercholesterolemic renal transplant recipient. Am J Kidney Dis. 2003;41:1088–1096. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

