Crystal engineering of HIV-1 reverse transcriptase for structure-based drug design
- PMID: 18676450
- PMCID: PMC2528191
- DOI: 10.1093/nar/gkn464
Crystal engineering of HIV-1 reverse transcriptase for structure-based drug design
Abstract
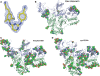
HIV-1 reverse transcriptase (RT) is a primary target for anti-AIDS drugs. Structures of HIV-1 RT, usually determined at approximately 2.5-3.0 A resolution, are important for understanding enzyme function and mechanisms of drug resistance in addition to being helpful in the design of RT inhibitors. Despite hundreds of attempts, it was not possible to obtain the structure of a complex of HIV-1 RT with TMC278, a nonnucleoside RT inhibitor (NNRTI) in advanced clinical trials. A systematic and iterative protein crystal engineering approach was developed to optimize RT for obtaining crystals in complexes with TMC278 and other NNRTIs that diffract X-rays to 1.8 A resolution. Another form of engineered RT was optimized to produce a high-resolution apo-RT crystal form, reported here at 1.85 A resolution, with a distinct RT conformation. Engineered RTs were mutagenized using a new, flexible and cost effective method called methylated overlap-extension ligation independent cloning. Our analysis suggests that reducing the solvent content, increasing lattice contacts, and stabilizing the internal low-energy conformations of RT are critical for the growth of crystals that diffract to high resolution. The new RTs enable rapid crystallization and yield high-resolution structures that are useful in designing/developing new anti-AIDS drugs.
Figures




References
-
- Coffin JM. HIV population dynamics in vivo-implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. - PubMed
-
- Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246:1155–1158. - PubMed
-
- Janssen PA, Lewi PJ, Arnold E, Daeyaert F, de Jonge M, Heeres J, Koymans L, Vinkers M, Guillemont J, Pasquier E, et al. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2, 6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine) J. Med. Chem. 2005;48:1901–1909. - PubMed
-
- Geretti AM. Shifting paradigms: the resistance profile of etravirine. J. Antimicrob. Chemother. 2008 [Epub ahead of print; doi:10.1093/jac/dkn248]; June 19, 2008. - PubMed
-
- Das K, Clark AD, Jr, Lewi PJ, Heeres J, de Jonge MR, Koymans LM, Vinkers HM, Daeyaert F, Ludovici DW, Kukla MJ, et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 2004;47:2550–2560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

