Calsequestrin-mediated mechanism for cellular calcium transient alternans
- PMID: 18676655
- PMCID: PMC2553104
- DOI: 10.1529/biophysj.108.130419
Calsequestrin-mediated mechanism for cellular calcium transient alternans
Erratum in
-
Calsequestrin-Mediated Mechanism for Cellular Calcium Transient Alternans.Biophys J. 2018 Apr 24;114(8):2024-2025. doi: 10.1016/j.bpj.2018.04.007. Biophys J. 2018. PMID: 29694878 Free PMC article. No abstract available.
Abstract
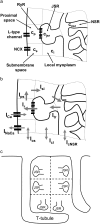
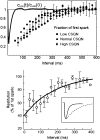
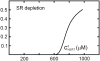
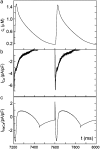
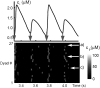
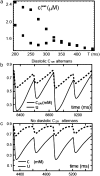
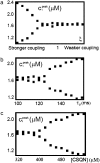
Intracellular calcium transient alternans (CTA) has a recognized role in arrhythmogenesis, but its origin is not yet fully understood. Recent models of CTA are based on a steep relationship between calcium release from the sarcoplasmic reticulum (SR) and its calcium load before release. This mechanism alone, however, does not explain recent observations of CTA without diastolic SR calcium content alternations. In addition, nanoscopic imaging of calcium dynamics has revealed that the elementary calcium release units of the SR can become refractory independently of their local calcium content. Here we show using a new physiologically detailed mathematical model of calcium cycling that luminal gating of the calcium release channels (RyRs) mediated by the luminal buffer calsequestrin (CSQN) can cause CTA independently of the steepness of the release-load relationship. In this complementary mechanism, CTA is caused by a beat-to-beat alternation in the number of refractory RyR channels and can occur with or without diastolic SR calcium content alternans depending on pacing conditions and uptake dynamics. The model has unique features, in that it treats a realistic number of spatially distributed and diffusively coupled dyads, each one with a realistic number of RyR channels, and that luminal CSQN buffering and gating is incorporated based on experimental data that characterizes the effect of the conformational state of CSQN on its buffering properties. In addition to reproducing observed features of CTA, this multiscale model is able to describe recent experiments in which CSQN expression levels were genetically altered as well as to reproduce nanoscopic measurements of spark restitution properties. The ability to link microscopic properties of the calcium release units to whole cell behavior makes this model a powerful tool to investigate the arrhythmogenic role of abnormal calcium handling in many pathological settings.
Figures





References
-
- Weiss, J. N., A. Karma, Y. Shiferaw, P. S. Chen, A. Garfinkel, and Z. Qu. 2006. From pulsus to pulseless: the saga of cardiac alternans. Circ. Res. 98:1244–1253. - PubMed
-
- Karma, A. 1994. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 4:461–472. - PubMed
-
- Pastore, J. M., S. D. Girouard, K. R. Laurita, F. G. Akar, and D. S. Rosenbaum. 1999. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 99:1385–1394. - PubMed
-
- Estes, N. A. M., G. Michaud, D. P. Zipes, N. ElSherif, F. J. Venditti, D. S. Rosenbaum, P. Albrecht, P. J. Wang, and R. J. Cohen. 1997. Electrical alternans during rest and exercise as predictors of vulnerability to ventricular arrhythmias. Am. J. Cardiol. 80:1314–1318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

