Interactions between SIRT1 and AP-1 reveal a mechanistic insight into the growth promoting properties of alumina (Al2O3) nanoparticles in mouse skin epithelial cells
- PMID: 18676681
- PMCID: PMC2722855
- DOI: 10.1093/carcin/bgn175
Interactions between SIRT1 and AP-1 reveal a mechanistic insight into the growth promoting properties of alumina (Al2O3) nanoparticles in mouse skin epithelial cells
Abstract
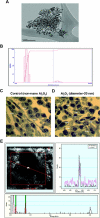
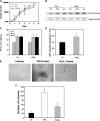
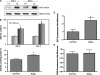
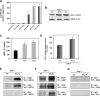
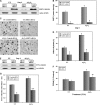
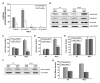
The physicochemical properties of nanomaterials differ from those of the bulk material of the same composition. However, little is known about the underlying effects of these particles in carcinogenesis. The purpose of this study was to determine the mechanisms involved in the carcinogenic properties of nanoparticles using aluminum oxide (Al(2)O(3)/alumina) nanoparticles as the prototype. Well-established mouse epithelial JB6 cells, sensitive to neoplastic transformation, were used as the experimental model. We demonstrate that alumina was internalized and maintained its physicochemical composition inside the cells. Alumina increased cell proliferation (53%), proliferating cell nuclear antigen (PCNA) levels, cell viability and growth in soft agar. The level of manganese superoxide dismutase, a key mitochondrial antioxidant enzyme, was elevated, suggesting a redox signaling event. In addition, the levels of reactive oxygen species and the activities of the redox sensitive transcription factor activator protein-1 (AP-1) and a longevity-related protein, sirtuin 1 (SIRT1), were increased. SIRT1 knockdown reduces DNA synthesis, cell viability, PCNA levels, AP-1 transcriptional activity and protein levels of its targets, JunD, c-Jun and BcL-xl, more than controls do. Immunoprecipitation studies revealed that SIRT1 interacts with the AP-1 components c-Jun and JunD but not with c-Fos. The results identify SIRT1 as an AP-1 modulator and suggest a novel mechanism by which alumina nanoparticles may function as a potential carcinogen.
Figures
References
-
- Xia T, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

