Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia
- PMID: 18676689
- PMCID: PMC2593505
- DOI: 10.1152/ajpheart.00301.2007
Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia
Abstract
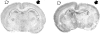
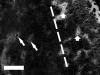
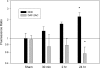
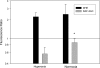
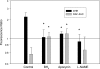
Decreased cerebral blood flow (CBF) has been observed following the resuscitation from neonatal hypoxic-ischemic injury, but its mechanism is not known. We address the hypothesis that reduced CBF is due to a change in nitric oxide (NO) and superoxide anion O(2)(-) balance secondary to endothelial NO synthase (eNOS) uncoupling with vascular injury. Wistar rats (7 day old) were subjected to cerebral hypoxia-ischemia by unilateral carotid occlusion under isoflurane anesthesia followed by hypoxia with hyperoxic or normoxic resuscitation. Expired CO(2) was determined during the period of hyperoxic or normoxic resuscitation. Laser-Doppler flowmetry was used with isoflurane anesthesia to monitor CBF, and cerebral perivascular NO and O(2)(-) were determined using fluorescent dyes with fluorescence microscopy. The effect of tetrahydrobiopterin supplementation on each of these measurements and the effect of apocynin and N(omega)-nitro-L-arginine methyl ester (L-NAME) administration on NO and O(2)(-) were determined. As a result, CBF in the ischemic cortex declined following the onset of resuscitation with 100% O(2) (hyperoxic resuscitation) but not room air (normoxic resuscitation). Expired CO(2) was decreased at the onset of resuscitation, but recovery was the same in normoxic and hyperoxic resuscitated groups. Perivascular NO-induced fluorescence intensity declined, and O(2)(-)-induced fluorescence increased in the ischemic cortex after hyperoxic resuscitation up to 24 h postischemia. L-NAME treatment reduced O(2)(-) relative to the nonischemic cortex. Apocynin treatment increased NO and reduced O(2)(-) relative to the nonischemic cortex. The administration of tetrahydrobiopterin following the injury increased perivascular NO, reduced perivascular O(2)(-), and increased CBF during hyperoxic resuscitation. These results demonstrate that reduced CBF follows hyperoxic resuscitation but not normoxic resuscitation after neonatal hypoxic-ischemic injury, accompanied by a reduction in perivascular production of NO and an increase in O(2)(-). The finding that tetrahydrobiopterin, apocynin, and L-NAME normalized radical production suggests that the uncoupling of perivascular NOS, probably eNOS, due to acquired relative tetrahydrobiopterin deficiency occurs after neonatal hypoxic-ischemic brain injury. It appears that both NOS uncoupling and the activation of NADPH oxidase participate in the changes of reactive oxygen concentrations seen in cerebral hypoxic-ischemic injury.
Figures



Comment in
-
To oxygenate or not to oxygenate--that is the question.Am J Physiol Heart Circ Physiol. 2008 Oct;295(4):H1371-2. doi: 10.1152/ajpheart.00880.2008. Epub 2008 Aug 15. Am J Physiol Heart Circ Physiol. 2008. PMID: 18708440 No abstract available.
References
-
- American Heart Association. Part 13: Neonatal Resuscitation Guildlines. Circulation 112: 188–195, 2005.
-
- Barbacanne MA, Souchard JP, Darblade B, Iliou JP, Nepveu F, Pipy B, Bayard F, Arnal JF. Detection of superoxide anion released extracellularly by endothelial cells using cytochrome c reduction, ESR, fluorescence and lucigenin-enhanced chemiluminescence techniques. Free Radic Biol Med 29: 388–396, 2000. - PubMed
-
- Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol 55: 253–258, 1994. - PubMed
-
- Channon KM, Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol 53: 515–524, 2002. - PubMed
-
- Channon KM Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14: 323–327, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

