Telomere maintenance in laser capture microdissection-purified Barrett's adenocarcinoma cells and effect of telomerase inhibition in vivo
- PMID: 18676772
- PMCID: PMC2705940
- DOI: 10.1158/1078-0432.CCR-08-0473
Telomere maintenance in laser capture microdissection-purified Barrett's adenocarcinoma cells and effect of telomerase inhibition in vivo
Abstract
Purpose: The aims of this study were to investigate telomere function in normal and Barrett's esophageal adenocarcinoma (BEAC) cells purified by laser capture microdissection and to evaluate the effect of telomerase inhibition in cancer cells in vitro and in vivo.
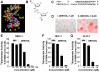
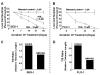
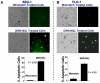
Experimental design: Epithelial cells were purified from surgically resected esophagi. Telomerase activity was measured by modified telomeric repeat amplification protocol and telomere length was determined by real-time PCR assay. To evaluate the effect of telomerase inhibition, adenocarcinoma cell lines were continuously treated with a specific telomerase inhibitor (GRN163L) and live cell number was determined weekly. Apoptosis was evaluated by Annexin labeling and senescence by beta-galactosidase staining. For in vivo studies, severe combined immunodeficient mice were s.c. inoculated with adenocarcinoma cells and following appearance of palpable tumors, injected i.p. with saline or GRN163L.
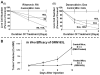
Results: Telomerase activity was significantly elevated whereas telomeres were shorter in BEAC cells relative to normal esophageal epithelial cells. The treatment of adenocarcinoma cells with telomerase inhibitor, GRN163L, led to loss of telomerase activity, reduction in telomere length, and growth arrest through induction of both the senescence and apoptosis. GRN163L-induced cell death could also be expedited by addition of the chemotherapeutic agents doxorubicin and ritonavir. Finally, the treatment with GRN163L led to a significant reduction in tumor volume in a subcutaneous tumor model.
Conclusions: We show that telomerase activity is significantly elevated whereas telomeres are shorter in BEAC and suppression of telomerase inhibits proliferation of adenocarcinoma cells both in vitro and in vivo.
Figures

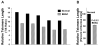

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

