A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans
- PMID: 18676815
- PMCID: PMC2492746
- DOI: 10.1101/gad.1692008
A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans
Abstract
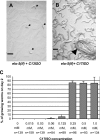
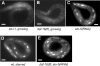
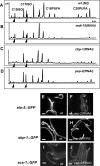
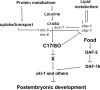
Growth and development of multicellular organisms are controlled by signaling systems that sense nutrition availability and metabolic status. We report a novel and surprising factor in Caenorhabditis elegans development, the monomethyl branched-chain fatty acid C17ISO, a product of leucine catabolism. We show here that C17ISO is an essential constituent in a novel mechanism that acts in parallel with the food-sensing DAF-2 (insulin receptor)/DAF-16 (FOXO) signaling pathway to promote post-embryonic development, and that the two pathways converge on a common target repressing cell cycle. We show that C17ISO homeostasis is regulated by a SREBP-1c-mediated feedback mechanism that is different from the SREBP-1c-mediated regulation of common fatty acid biosynthesis, as well as by peptide uptake and transport. Our data suggest that C17ISO may act as a chemical/nutritional factor in a mechanism that regulates post-embryonic development in response to the metabolic state of the organism.
Figures


References
-
- Baugh L.R., Sternberg P.W. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 2006;16:780–785. - PubMed
-
- Fukuyama M., Rougvie A.E., Rothman J.H. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr. Biol. 2006;16:773–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
