Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer
- PMID: 18676839
- PMCID: PMC2597340
- DOI: 10.1158/0008-5472.CAN-08-0144
Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer
Abstract
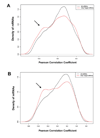
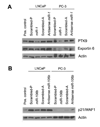
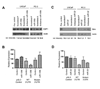
MicroRNAs are small noncoding RNAs that regulate the expression of protein-coding genes. To evaluate the involvement of microRNAs in prostate cancer, we determined genome-wide expression of microRNAs and mRNAs in 60 primary prostate tumors and 16 nontumor prostate tissues. The mRNA analysis revealed that key components of microRNA processing and several microRNA host genes, e.g., MCM7 and C9orf5, were significantly up-regulated in prostate tumors. Consistent with these findings, tumors expressed the miR-106b-25 cluster, which maps to intron 13 of MCM7, and miR-32, which maps to intron 14 of C9orf5, at significantly higher levels than nontumor prostate. The expression levels of other microRNAs, including a number of miR-106b-25 cluster homologues, were also altered in prostate tumors. Additional differences in microRNA abundance were found between organ-confined tumors and those with extraprostatic disease extension. Lastly, we found evidence that some microRNAs are androgen-regulated and that tumor microRNAs influence transcript abundance of protein-coding target genes in the cancerous prostate. In cell culture, E2F1 and p21/WAF1 were identified as targets of miR-106b, Bim of miR-32, and exportin-6 and protein tyrosine kinase 9 of miR-1. In summary, microRNA expression becomes altered with the development and progression of prostate cancer. Some of these microRNAs regulate the expression of cancer-related genes in prostate cancer cells.
Figures
References
-
- Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. - PubMed
-
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat.Rev.Genet. 2004;5:522–531. - PubMed
-
- Yu J, Wang F, Yang GH, et al. Human microRNA clusters: genomic organization and expression profile in leukemia cell lines. Biochem Biophys.Res Commun. 2006;349:59–68. - PubMed
-
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat.Genet. 2007;39:673–677. - PubMed
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat.Rev.Cancer. 2006;6:857–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

