Reciprocal relationship between O6-methylguanine-DNA methyltransferase P140K expression level and chemoprotection of hematopoietic stem cells
- PMID: 18676840
- PMCID: PMC4337843
- DOI: 10.1158/0008-5472.CAN-08-0320
Reciprocal relationship between O6-methylguanine-DNA methyltransferase P140K expression level and chemoprotection of hematopoietic stem cells
Abstract
Retroviral-mediated delivery of the P140K mutant O(6)-methylguanine-DNA methyltransferase (MGMT(P140K)) into hematopoietic stem cells (HSC) has been proposed as a means to protect against dose-limiting myelosuppressive toxicity ensuing from chemotherapy combining O(6)-alkylating agents (e.g., temozolomide) with pseudosubstrate inhibitors (such as O(6)-benzylguanine) of endogenous MGMT. Because detoxification of O(6)-alkylguanine adducts by MGMT is stoichiometric, it has been suggested that higher levels of MGMT will afford better protection to gene-modified HSC. However, accomplishing this goal would potentially be in conflict with current efforts in the gene therapy field, which aim to incorporate weaker enhancer elements to avoid insertional mutagenesis. Using a panel of self-inactivating gamma-retroviral vectors that express a range of MGMT(P140K) activity, we show that MGMT(P140K) expression by weaker cellular promoter/enhancers is sufficient for in vivo protection/selection following treatment with O(6)-benzylguanine/temozolomide. Conversely, the highest level of MGMT(P140K) activity did not promote efficient in vivo protection despite mediating detoxification of O(6)-alkylguanine adducts. Moreover, very high expression of MGMT(P140K) was associated with a competitive repopulation defect in HSC. Mechanistically, we show a defect in cellular proliferation associated with elevated expression of MGMT(P140K), but not wild-type MGMT. This proliferation defect correlated with increased localization of MGMT(P140K) to the nucleus/chromatin. These data show that very high expression of MGMT(P140K) has a deleterious effect on cellular proliferation, engraftment, and chemoprotection. These studies have direct translational relevance to ongoing clinical gene therapy studies using MGMT(P140K), whereas the novel mechanistic findings are relevant to the basic understanding of DNA repair by MGMT.
Conflict of interest statement
Figures
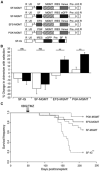
 , EFS-MGMT;
, EFS-MGMT;
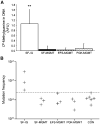
 , PGK-MGMT. **, P < 0.01, versus all other groups (Student's t test). B, absolute mutation frequency in transduced bone marrow. At 6 mo posttreatment, the surviving mice were sacrificed. Bone marrow was harvested and LacZ reporter plasmids were subsequently rescued from the resulting genomic DNA as described in Materials and Methods. The mutation frequency in the rescued reporter plasmids was then determined. Each cross represents the mutation frequency determined for bone marrow from a single mouse. Dashed line, the upper range of mutation frequency in nontreated controls (CON).
, PGK-MGMT. **, P < 0.01, versus all other groups (Student's t test). B, absolute mutation frequency in transduced bone marrow. At 6 mo posttreatment, the surviving mice were sacrificed. Bone marrow was harvested and LacZ reporter plasmids were subsequently rescued from the resulting genomic DNA as described in Materials and Methods. The mutation frequency in the rescued reporter plasmids was then determined. Each cross represents the mutation frequency determined for bone marrow from a single mouse. Dashed line, the upper range of mutation frequency in nontreated controls (CON).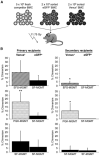
 ), or PGK-MGMT–transduced (
), or PGK-MGMT–transduced (
 ) cells in the peripheral blood. Columns, mean of eight mice per group; bars, SD. **, P < 0.01; *, P < 0.05, versus the SF-MGMT GFP+ graft in the same recipients. SF-MGMT Venus+ transduced bone marrow is used to control for bias due to type of fluorochrome used.
) cells in the peripheral blood. Columns, mean of eight mice per group; bars, SD. **, P < 0.01; *, P < 0.05, versus the SF-MGMT GFP+ graft in the same recipients. SF-MGMT Venus+ transduced bone marrow is used to control for bias due to type of fluorochrome used.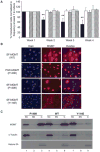
 , EFS-MGMT;
, EFS-MGMT;
 , PGK-MGMT;
, PGK-MGMT;
 , SF-MGMT vector containing WT cDNA. Columns, mean of three to nine independent experiments; bars, SD. **, P < 0.01, compared with other groups (Student's t test). B, NIH 3T3 fibroblast cells were transduced with the indicated retroviral vectors. Cells were fixed and stained with DAPI and anti-MGMT antibody. Photomicrographs of fixed and stained cells were at ×400 magnification. Bar, 9.75 μm. C, 32D cells were transduced with SF-MGMT vectors containing the WT MGMT sequence, the P140K mutant, or the Y114E mutant as a non–DNA-binding control. Transduced cells were isolated by flow sorting and cells were lysed and cellular constituents separated as described in Materials and Methods before immunoblot with the indicated antibody. SC, cytoplasmic and soluble cytoplasmic fraction; SN, soluble nuclear fraction; C, chromatin/nuclear matrix–bound fraction. Lanes 1 to 3, P140K; lanes 4 to 6, WT; lanes 7 to 9, Y114E.
, SF-MGMT vector containing WT cDNA. Columns, mean of three to nine independent experiments; bars, SD. **, P < 0.01, compared with other groups (Student's t test). B, NIH 3T3 fibroblast cells were transduced with the indicated retroviral vectors. Cells were fixed and stained with DAPI and anti-MGMT antibody. Photomicrographs of fixed and stained cells were at ×400 magnification. Bar, 9.75 μm. C, 32D cells were transduced with SF-MGMT vectors containing the WT MGMT sequence, the P140K mutant, or the Y114E mutant as a non–DNA-binding control. Transduced cells were isolated by flow sorting and cells were lysed and cellular constituents separated as described in Materials and Methods before immunoblot with the indicated antibody. SC, cytoplasmic and soluble cytoplasmic fraction; SN, soluble nuclear fraction; C, chromatin/nuclear matrix–bound fraction. Lanes 1 to 3, P140K; lanes 4 to 6, WT; lanes 7 to 9, Y114E.
 , EFS-MGMT;
, EFS-MGMT;
 , PGK-MGMT *, P < 0.05; **, P < 0.01, compared with SF-MGMT–transduced group. B, lysate from transduced and sorted 32D cells described in A was prepared at 6 h postinduction. These lysates were then subject to immunoblot and probed with the indicated antibodies. Lysate from starved (S) nontransduced cells is shown as control to illustrate the level of p27 in noninduced cells.
, PGK-MGMT *, P < 0.05; **, P < 0.01, compared with SF-MGMT–transduced group. B, lysate from transduced and sorted 32D cells described in A was prepared at 6 h postinduction. These lysates were then subject to immunoblot and probed with the indicated antibodies. Lysate from starved (S) nontransduced cells is shown as control to illustrate the level of p27 in noninduced cells.References
-
- Milsom M, Schambach A, Williams DA, Baum C. Chemoprotective gene delivery. In: Harington KJ, Vile RG, Pandha H, editors. Viral therapy of cancer. Indianapolis (IN): Wiley Press; 2008. pp. 377–91.
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Phillips GL, Wolff SN, Fay JW, et al. Intensive 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) monochemotherapy and autologous marrow transplantation for malignant glioma. J Clin Oncol. 1986;4:639–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

