Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons
- PMID: 18676868
- PMCID: PMC2591093
- DOI: 10.1158/0008-5472.CAN-08-1173
Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons
Abstract
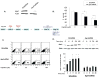
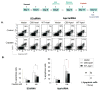
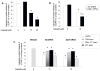
Peripheral neuropathy is one of the major side effects of the anticancer drug cisplatin. Although previous work suggests that this neuropathy correlates with formation of DNA adducts in sensory neurons, growing evidence suggests that cisplatin also increases the generation of reactive oxygen species (ROS), which could cause DNA damage. Apurinic/apyrimidinic endonuclease/redox factor-1 (Ape1/Ref-1) is a multifunctional protein involved in DNA base excision repair of oxidative DNA damage and in redox regulation of a number of transcription factors. Therefore, we asked whether altering Ape1 functions would influence cisplatin-induced neurotoxicity. Sensory neurons in culture were exposed to cisplatin for 24 hours and several end points of toxicity were measured, including production of ROS, cell death, apoptosis, and release of the immunoreactive calcitonin gene-related peptide (iCGRP). Reducing expression of Ape1 in neuronal cultures using small interfering RNA (siRNA) enhances cisplatin-induced cell killing, apoptosis, ROS generation, and cisplatin-induced reduction in iCGRP release. Overexpressing wild-type Ape1 attenuates all the toxic effects of cisplatin in cells containing normal endogenous levels of Ape1 and in cells with reduced Ape1 levels after Ape1siRNA treatment. Overexpressing the redox deficient/repair competent C65-Ape1 provides partial rescue, whereas the repair-deficient Ape1 (N226A + R177A) does not protect neurons from cisplatin toxicity. We also observe an increase in phosphorylation of p53 after a decrease in Ape1 levels in sensory neuronal cultures. These results strongly support the notion that Ape1 is a potential translational target such that protecting Ape1 levels and particularly its DNA repair function could reduce peripheral neuropathy in patients undergoing cisplatin treatment.
Figures


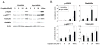
References
-
- Manju K, Muralikrishna B, Parnaik VK. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J Cell Sci. 2006;119:2704–14. - PubMed
-
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. - PubMed
-
- McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–13. - PubMed
-
- Holmes J, Stanko J, Varchenko M, et al. Comparative neurotoxicity of oxaliplatin, cisplatin, and ormaplatin in a Wistar rat model. Toxicol Sci. 1998;46:342–51. - PubMed
-
- Wu F, Lin X, Okuda T, Howell SB. DNA polymerase zeta regulates cisplatin cytotoxicity, mutagenicity, and the rate of development of cisplatin resistance. Cancer Res. 2004;64:8029–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

