Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection
- PMID: 18676881
- PMCID: PMC2565907
- DOI: 10.1128/AAC.00654-08
Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection
Abstract
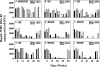
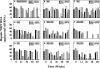
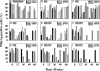
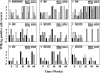
Adefovir dipivoxil (ADV) and tenofovir disoproxil fumarate (TDF) are nucleotide analogs that inhibit the replication of wild-type hepatitis B virus (HBV) and lamivudine (3TC)-resistant virus in HBV-infected patients, including those who are coinfected with human immunodeficiency virus. The combination of ADV or TDF with other nucleoside analogs is a proposed strategy for managing antiviral drug resistance during the treatment of chronic HBV infection. The antiviral effect of oral ADV or TDF, alone or in combination with 3TC or emtricitabine (FTC), against chronic woodchuck hepatitis virus (WHV) infection was evaluated in a placebo-controlled study in the woodchuck, an established and predictive model for antiviral therapy. Once-daily treatment for 48 weeks with ADV plus 3TC or TDF plus FTC significantly reduced serum WHV viremia levels from the pretreatment level by 6.2 log(10) and 6.1 log(10) genome equivalents/ml serum, respectively, followed by TDF plus 3TC (5.6 log(10) genome equivalents/ml), ADV alone (4.8 log(10) genome equivalents/ml), ADV plus FTC (one survivor) (4.4 log(10) genome equivalents/ml), TDF alone (2.9 log(10) genome equivalents/ml), 3TC alone (2.7 log(10) genome equivalents/ml), and FTC alone (2.0 log(10) genome equivalents/ml). Individual woodchucks across all treatment groups also demonstrated pronounced declines in serum WHV surface antigen, characteristically accompanied by declines in hepatic WHV replication and the hepatic expression of WHV antigens. Most woodchucks had prompt recrudescence of WHV replication after drug withdrawal, but individual woodchucks across treatment groups had sustained effects. No signs of toxicity were observed for any of the drugs or drug combinations administered. In conclusion, the oral administration of 3TC, FTC, ADV, and TDF alone and in combination was safe and effective in the woodchuck model of HBV infection.
Figures





Similar articles
-
Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection.Antimicrob Agents Chemother. 2005 Jul;49(7):2720-8. doi: 10.1128/AAC.49.7.2720-2728.2005. Antimicrob Agents Chemother. 2005. PMID: 15980342 Free PMC article.
-
Suppression of lamivudine-resistant B-domain mutants by adefovir dipivoxil in the woodchuck hepatitis virus model.Antiviral Res. 2004 Aug;63(2):115-21. doi: 10.1016/j.antiviral.2004.03.005. Antiviral Res. 2004. PMID: 15302140
-
Similar evolution of hepatitis B virus quasispecies in patients with incomplete adefovir response receiving tenofovir/emtricitabine combination or tenofovir monotherapy.J Hepatol. 2013 Oct;59(4):684-95. doi: 10.1016/j.jhep.2013.05.038. Epub 2013 Jun 3. J Hepatol. 2013. PMID: 23742912 Clinical Trial.
-
[New drugs of treatment of patients with chronic hepatitis B].Nihon Rinsho. 2012 Apr;70(4):660-3. Nihon Rinsho. 2012. PMID: 22568150 Review. Japanese.
-
The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection.World J Gastroenterol. 2007 Jan 7;13(1):104-24. doi: 10.3748/wjg.v13.i1.104. World J Gastroenterol. 2007. PMID: 17206759 Free PMC article. Review.
Cited by
-
Antiviral Efficacy and Host Immune Response Induction during Sequential Treatment with SB 9200 Followed by Entecavir in Woodchucks.PLoS One. 2017 Jan 5;12(1):e0169631. doi: 10.1371/journal.pone.0169631. eCollection 2017. PLoS One. 2017. PMID: 28056062 Free PMC article.
-
Treatment with the Immunomodulator AIC649 in Combination with Entecavir Produces Antiviral Efficacy in the Woodchuck Model of Chronic Hepatitis B.Viruses. 2021 Apr 9;13(4):648. doi: 10.3390/v13040648. Viruses. 2021. PMID: 33918831 Free PMC article.
-
Antiviral Efficacy and Host Innate Immunity Associated with SB 9200 Treatment in the Woodchuck Model of Chronic Hepatitis B.PLoS One. 2016 Aug 23;11(8):e0161313. doi: 10.1371/journal.pone.0161313. eCollection 2016. PLoS One. 2016. PMID: 27552102 Free PMC article.
-
Toll-Like Receptor 7 Agonist RG7854 Mediates Therapeutic Efficacy and Seroconversion in Woodchucks With Chronic Hepatitis B.Front Immunol. 2022 May 23;13:884113. doi: 10.3389/fimmu.2022.884113. eCollection 2022. Front Immunol. 2022. PMID: 35677037 Free PMC article.
-
Intrahepatic Transcriptional Signature Associated with Response to Interferon-α Treatment in the Woodchuck Model of Chronic Hepatitis B.PLoS Pathog. 2015 Sep 9;11(9):e1005103. doi: 10.1371/journal.ppat.1005103. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26352406 Free PMC article.
References
-
- Akyildiz, M., F. Gunsar, G. Ersoz, Z. Karasu, T. Ilter, Y. Batur, and U. Akarca. 2007. Adefovir dipivoxil alone or in combination with lamivudine for three months in patients with lamivudine resistant compensated chronic hepatitis B. Dig. Dis. Sci. 52:3444-3447. - PubMed
-
- Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. - PubMed
-
- Benhamou, Y., M. Bochet, V. Thibault, V. Calvez, M. H. Fievet, P. Vig, C. S. Gibbs, C. Brosgart, J. Fry, H. Namini, C. Katlama, and T. Poynard. 2001. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet 358:718-723. - PubMed
-
- Benhamou, Y., V. Thibault, P. Vig, V. Calvez, A. G. Marcelin, M. H. Fievet, G. Currie, C. G. Chang, L. Biao, S. Xiong, C. Brosgart, and T. Poynard. 2006. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J. Hepatol. 44:62-67. - PubMed
-
- Benhamou, Y., R. Tubiana, and V. Thibault. 2003. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N. Engl. J. Med. 348:177-178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

