Low penetration of oseltamivir and its carboxylate into cerebrospinal fluid in healthy Japanese and Caucasian volunteers
- PMID: 18676886
- PMCID: PMC2565879
- DOI: 10.1128/AAC.00327-08
Low penetration of oseltamivir and its carboxylate into cerebrospinal fluid in healthy Japanese and Caucasian volunteers
Abstract
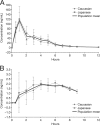
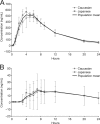
Oseltamivir is a potent, well-tolerated antiviral for the treatment and prophylaxis of influenza. Although no relationship with treatment could be demonstrated, recent reports of abnormal behavior in young individuals with influenza who were receiving oseltamivir have generated renewed interest in the central nervous system (CNS) tolerability of oseltamivir. This single-center, open-label study explored the pharmacokinetics of oseltamivir and oseltamivir carboxylate (OC) in the plasma and cerebrospinal fluid (CSF) of healthy adult volunteers over a 24-hour interval to determine the CNS penetration of both these compounds. Four Japanese and four Caucasian males were enrolled in the study. Oseltamivir and OC concentrations in CSF were low (mean of observed maximum concentrations [C(max)], 2.4 ng/ml [oseltamivir] and 19.0 ng/ml [OC]) versus those in plasma (mean C(max), 115 ng/ml [oseltamivir] and 544 ng/ml [OC]), with corresponding C(max) CSF/plasma ratios of 2.1% (oseltamivir) and 3.5% (OC). Overall exposure to oseltamivir and OC in CSF was also comparatively low versus that in plasma (mean area under the concentration-time curve CSF/plasma ratio, 2.4% [oseltamivir] and 2.9% [OC]). No gross differences in the pharmacokinetics of oseltamivir or OC were observed between the Japanese and Caucasian subjects. Oseltamivir was well tolerated. This demonstrates that the CNS penetration of oseltamivir and OC is low in Japanese and Caucasian adults. Emerging data support the idea that oseltamivir and OC have limited potential to induce or exacerbate CNS adverse events in individuals with influenza. A disease- rather than drug-related effect appears likely.
Figures
References
-
- Bleasby, K., L. A. Hall, J. L. Perry, H. W. Mohrenweiser, and J. B. Pritchard. 2005. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J. Pharmacol. Exp. Ther. 314:923-931. - PubMed
-
- Ereshefsky, L., S. S. Jhee, M. T. Leibowitz, S. Moran, M. Yen, and L. Gertsik. 2006. Demonstrating proof of principle and finding the right dose: the role of CSF ‘Dynabridging’ studies. Abstr. 25th Bienn. Congr. Coll. Int. Neuro-Psychopharmacol. (CINP), Chicago, IL.
-
- Fujita, T., C. Brown, E. J. Carlson, T. Taylor, M. de la Cruz, S. J. Johns, D. Stryke, M. Kawamoto, K. Fujita, R. Castro, C. W. Chen, E. T. Lin, C. M. Brett, E. G. Burchard, T. E. Ferrin, C. C. Huang, M. K. Leabman, and K. M. Giacomini. 2005. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenet. Genomics 15:201-209. - PubMed
-
- Fuke, C., Y. Ihama, and T. Miyazaki. 2008. Analysis of oseltamivir active metabolite, oseltamivir carboxylate, in biological materials by HPLC-UV in a case of death following ingestion of Tamiflu. J. Leg. Med. (Tokyo) 10:83-87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

