A member of the Whirly family is a multifunctional RNA- and DNA-binding protein that is essential for chloroplast biogenesis
- PMID: 18676978
- PMCID: PMC2532728
- DOI: 10.1093/nar/gkn492
A member of the Whirly family is a multifunctional RNA- and DNA-binding protein that is essential for chloroplast biogenesis
Abstract
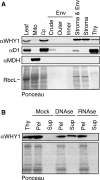
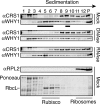
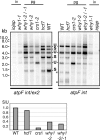
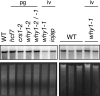
'Whirly' proteins comprise a plant-specific protein family whose members have been described as DNA-binding proteins that influence nuclear transcription and telomere maintenance, and that associate with nucleoids in chloroplasts and mitochondria. We identified the maize WHY1 ortholog among proteins that coimmunoprecipitate with CRS1, which promotes the splicing of the chloroplast atpF group II intron. ZmWHY1 localizes to the chloroplast stroma and to the thylakoid membrane, to which it is tethered by DNA. Genome-wide coimmunoprecipitation assays showed that ZmWHY1 in chloroplast extract is associated with DNA from throughout the plastid genome and with a subset of plastid RNAs that includes atpF transcripts. Furthermore, ZmWHY1 binds both RNA and DNA in vitro. A severe ZmWhy1 mutant allele conditions albino seedlings lacking plastid ribosomes; these exhibit the altered plastid RNA profile characteristic of ribosome-less plastids. Hypomorphic ZmWhy1 mutants exhibit reduced atpF intron splicing and a reduced content of plastid ribosomes; aberrant 23S rRNA metabolism in these mutants suggests that a defect in the biogenesis of the large ribosomal subunit underlies the ribosome deficiency. However, these mutants contain near normal levels of chloroplast DNA and RNAs, suggesting that ZmWHY1 is not directly required for either DNA replication or for global plastid transcription.
Figures





Similar articles
-
Chloroplast RH3 DEAD box RNA helicases in maize and Arabidopsis function in splicing of specific group II introns and affect chloroplast ribosome biogenesis.Plant Physiol. 2012 Jul;159(3):961-74. doi: 10.1104/pp.112.197525. Epub 2012 May 10. Plant Physiol. 2012. PMID: 22576849 Free PMC article.
-
CRS1, a chloroplast group II intron splicing factor, promotes intron folding through specific interactions with two intron domains.Plant Cell. 2005 Jan;17(1):241-55. doi: 10.1105/tpc.104.027516. Epub 2004 Dec 14. Plant Cell. 2005. PMID: 15598799 Free PMC article.
-
An mTERF domain protein functions in group II intron splicing in maize chloroplasts.Nucleic Acids Res. 2014 Apr;42(8):5033-42. doi: 10.1093/nar/gku112. Epub 2014 Feb 5. Nucleic Acids Res. 2014. PMID: 24500208 Free PMC article.
-
Large-scale genetic analysis of chloroplast biogenesis in maize.Biochim Biophys Acta. 2015 Sep;1847(9):1004-16. doi: 10.1016/j.bbabio.2015.02.014. Epub 2015 Feb 26. Biochim Biophys Acta. 2015. PMID: 25725436 Review.
-
Function of chloroplast RNA-binding proteins.Cell Mol Life Sci. 2011 Mar;68(5):735-48. doi: 10.1007/s00018-010-0523-3. Epub 2010 Sep 17. Cell Mol Life Sci. 2011. PMID: 20848156 Free PMC article. Review.
Cited by
-
Dual targeting and retrograde translocation: regulators of plant nuclear gene expression can be sequestered by plastids.Int J Mol Sci. 2012;13(9):11085-11101. doi: 10.3390/ijms130911085. Epub 2012 Sep 6. Int J Mol Sci. 2012. PMID: 23109840 Free PMC article. Review.
-
Genome-wide identification, phylogenetic, and expression analysis under abiotic stress conditions of Whirly (WHY) gene family in Medicago sativa L.Sci Rep. 2022 Nov 4;12(1):18676. doi: 10.1038/s41598-022-22658-3. Sci Rep. 2022. PMID: 36333411 Free PMC article.
-
The RAD52-like protein ODB1 is required for the efficient excision of two mitochondrial introns spliced via first-step hydrolysis.Nucleic Acids Res. 2015 Jul 27;43(13):6500-10. doi: 10.1093/nar/gkv540. Epub 2015 Jun 5. Nucleic Acids Res. 2015. PMID: 26048959 Free PMC article.
-
ZmpTAC12 binds single-stranded nucleic acids and is essential for accumulation of the plastid-encoded polymerase complex in maize.New Phytol. 2015 May;206(3):1024-1037. doi: 10.1111/nph.13248. Epub 2015 Jan 19. New Phytol. 2015. PMID: 25599833 Free PMC article.
-
Function of plastid sigma factors in higher plants: regulation of gene expression or just preservation of constitutive transcription?Plant Mol Biol. 2011 Jul;76(3-5):235-49. doi: 10.1007/s11103-010-9714-4. Epub 2010 Nov 25. Plant Mol Biol. 2011. PMID: 21107995
References
-
- Marchfelder A, Binder S. Plastid and plant mitochondrial RNA processing and RNA stability. In: Daniell H, Chase C, editors. Molecular Biology and Biotechnology of Plant Organelles. Kluwer Academic Publishers: Dordrecht, The Netherlands; 2004. pp. 261–294.
-
- Nickelsen J. Chloroplast RNA-binding proteins. Curr. Genet. 2003;43:392–399. - PubMed
-
- Schwacke R, Fischer K, Ketelsen B, Krupinska K, Krause K. Comparative survey of plastid and mitochondrial targeting properties of transcription factors in Arabidopsis and rice. Mol. Genet. Genomics. 2007;277:631–646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

