FERM control of FAK function: implications for cancer therapy
- PMID: 18677107
- PMCID: PMC2574722
- DOI: 10.4161/cc.6367
FERM control of FAK function: implications for cancer therapy
Abstract
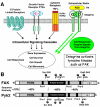
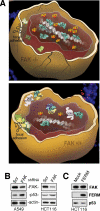
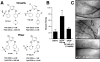
Integrins are transmembrane receptors that bind to extracellular matrix proteins and convey anchorage-dependent signals regulating normal cell proliferation. Integrin signals within the tumor micro-environment also impact cancer cell survival and invasion during tumor progression. These integrin-associated signaling events are transduced in part through the activation of non-receptor protein-tyrosine kinases. Focal adhesion kinase (FAK) is activated by beta-subunit integrins in both normal and transformed cells. As genetic inactivation of beta1 integrin or FAK yield early embryonic lethal phenotypes associated with decreased cell proliferation, and dominant-negative inhibition of FAK can cause increased cell apoptosis, there is a concern that FAK inhibition may have cytotoxic effects on cell growth or survival. However, FAK-specific small molecule inhibitors do not directly impact cell growth in culture, but yet show potent anti-tumor growth effects in vivo. Additionally, recent studies have shed new insight into the FAK kinase-independent regulation of cell proliferation and survival mediated by the FAK N-terminal FERM (band 4.1, ezrin, radixin, moesin homology) domain. Herein, we review the role of the FAK FERM domain in both the intrinsic regulation of FAK kinase activity and how FERM-mediated nuclear localization of FAK promotes enhanced cell survival through the inhibition of tumor suppressor p53 activation during development and under conditions of cellular stress. As we find that FAK FERM-mediated regulation of p53 occurs in human carcinoma cells, elevated FAK expression in tumors may promote both kinase-dependent and -independent survival mechanisms. We discuss how the pharmacological inhibition of FAK kinase activity may impact tumor progression through combined effects of blocking both tumor- and stromal-associated signaling regulating neo-vascularization.
Figures
Similar articles
-
Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation.Mol Cell. 2008 Jan 18;29(1):9-22. doi: 10.1016/j.molcel.2007.11.031. Mol Cell. 2008. PMID: 18206965 Free PMC article.
-
Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival.J Biol Chem. 2010 Jan 15;285(3):1743-53. doi: 10.1074/jbc.M109.064212. Epub 2009 Oct 30. J Biol Chem. 2010. PMID: 19880522 Free PMC article.
-
Phosphorylation of focal adhesion kinase on tyrosine 194 by Met leads to its activation through relief of autoinhibition.Oncogene. 2011 Jan 13;30(2):153-66. doi: 10.1038/onc.2010.398. Epub 2010 Aug 30. Oncogene. 2011. PMID: 20802513
-
Focal adhesion kinase as potential target for cancer therapy (Review).Oncol Rep. 2009 Nov;22(5):973-9. doi: 10.3892/or_00000524. Oncol Rep. 2009. PMID: 19787209 Review.
-
Focal adhesion kinase and its potential involvement in tumor invasion and metastasis.Head Neck. 1998 Dec;20(8):745-52. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. Head Neck. 1998. PMID: 9790298 Review.
Cited by
-
Inhibiting focal adhesion kinase: A potential target for enhancing therapeutic efficacy in colorectal cancer therapy.World J Gastrointest Oncol. 2018 Oct 15;10(10):290-292. doi: 10.4251/wjgo.v10.i10.290. World J Gastrointest Oncol. 2018. PMID: 30364839 Free PMC article.
-
Inhibition of cell migration by focal adhesion kinase: Time-dependent difference in integrin-induced signaling between endothelial and hepatoblastoma cells.Int J Mol Med. 2018 May;41(5):2573-2588. doi: 10.3892/ijmm.2018.3512. Epub 2018 Feb 23. Int J Mol Med. 2018. PMID: 29484384 Free PMC article.
-
Protons make tumor cells move like clockwork.Pflugers Arch. 2009 Sep;458(5):981-92. doi: 10.1007/s00424-009-0677-8. Epub 2009 May 13. Pflugers Arch. 2009. PMID: 19437033 Review.
-
Regulation of blood-testis barrier dynamics by focal adhesion kinase (FAK): an unexpected turn of events.Cell Cycle. 2009 Nov 1;8(21):3493-9. doi: 10.4161/cc.8.21.9833. Epub 2009 Nov 17. Cell Cycle. 2009. PMID: 19823026 Free PMC article.
-
Low concentrations of saracatinib promote definitive endoderm differentiation through inhibition of FAK-YAP signaling axis.Cell Commun Signal. 2024 May 30;22(1):300. doi: 10.1186/s12964-024-01679-7. Cell Commun Signal. 2024. PMID: 38816763 Free PMC article.
References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. - PubMed
-
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. - PubMed
-
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–71. - PubMed
-
- Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–39. - PubMed
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
