ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation
- PMID: 18677313
- PMCID: PMC2675613
- DOI: 10.1038/ng.199
ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation
Abstract
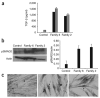
Geleophysic dysplasia is an autosomal recessive disorder characterized by short stature, brachydactyly, thick skin and cardiac valvular anomalies often responsible for an early death. Studying six geleophysic dysplasia families, we first mapped the underlying gene to chromosome 9q34.2 and identified five distinct nonsense and missense mutations in ADAMTSL2 (a disintegrin and metalloproteinase with thrombospondin repeats-like 2), which encodes a secreted glycoprotein of unknown function. Functional studies in HEK293 cells showed that ADAMTSL2 mutations lead to reduced secretion of the mutated proteins, possibly owing to the misfolding of ADAMTSL2. A yeast two-hybrid screen showed that ADAMTSL2 interacts with latent TGF-beta-binding protein 1. In addition, we observed a significant increase in total and active TGF-beta in the culture medium as well as nuclear localization of phosphorylated SMAD2 in fibroblasts from individuals with geleophysic dysplasia. These data suggest that ADAMTSL2 mutations may lead to a dysregulation of TGF-beta signaling and may be the underlying mechanism of geleophysic dysplasia.
Figures

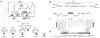


References
-
- Spranger JW, Gilbert EF, Tuffli GA, Rossiter FP, Opitz JM. Geleophysic dwarfism–a “focal” mucopolysaccharidosis? Lancet. 1971;2:97–98. - PubMed
-
- Pontz BF, et al. Clinical and ultrastructural findings in three patients with geleophysic dysplasia. Am J Med Genet. 1996;63:50–54. - PubMed
-
- Shohat M, et al. Geleophysic dysplasia: a storage disorder affecting the skin, bone, liver, heart, and trachea. J Pediatr. 1990;117:227–232. - PubMed
-
- Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–985. - PubMed
-
- Hirohata S, et al. Punctin, a novel ADAMTS-like molecule, ADAMTSL-1, in extracellular matrix. J Biol Chem. 2002;277:12182–12189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

