Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate
- PMID: 18677583
- PMCID: PMC2788987
- DOI: 10.1007/s12031-008-9122-2
Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate
Abstract
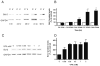
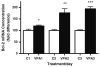
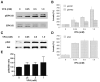
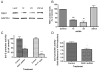
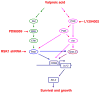
Valproate, an anticonvulsant and mood stabilizer, up-regulates Bcl-2, a neurotrophic/neuroprotective protein. In this study, we investigated the molecular mechanism through which Bcl-2 is up-regulated by valproate using cultured human neuron-like cells. Valproate, within therapeutically relevant ranges, induced time- and concentration-dependent up-regulations of both Bcl-2 messenger RNA and protein implicating an underlying gene transcriptional-mediated mechanism. Bcl-2 up-regulations were associated with ERK1/2 and PI3K pathway activations and elevated levels of activated phospho-RSK and phospho-CREB, convergent targets of the ERK1/2 and PI3K pathways. Valproate increased transcriptional activity of a human bcl-2 promoter-reporter gene construct. This effect was attenuated, but not blocked, by mutation of a CREB DNA binding site, a CRE site in the human bcl-2 promoter sequence. ERK and/or PI3K pathway inhibitors and RSK1 small hairpin RNA knockdown reduced, but did not abolish, baseline and valproate-induced promoter activities and lowered Bcl-2 protein levels. These data collectively suggest that valproate induces Bcl-2 regulation partially through activations of the ERK and PI3K cascades and their convergent kinase, RSK, although other unknown mechanism(s) are likely involved. Given the known roles of Bcl-2 in the central nervous system, the current findings offer a partial yet complex molecular mechanistic explanation for the known neurobiological effects of valproate including neurite growth, neuronal survival, and neurogenesis.
Figures


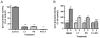

References
-
- Atack JR, Prior AM, Fletcher SR, Quirk K, McKernan R, Ragan CI. Effects of L-690,488, a prodrug of the bisphosphonate inositol monophosphatase inhibitor L-690,330, on phosphatidylinositol cycle markers. The Journal of Pharmacology and Experimental Therapeutics. 1994;270:70–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

