Ectopic expression of LIM-nebulette (LASP2) reveals roles in cell migration and spreading
- PMID: 18677772
- PMCID: PMC2603443
- DOI: 10.1002/cm.20304
Ectopic expression of LIM-nebulette (LASP2) reveals roles in cell migration and spreading
Abstract
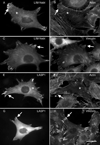
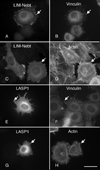
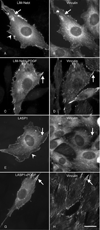
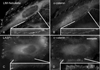
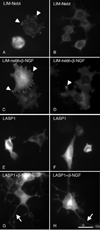
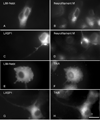
LIM-nebulette (LASP2) is a small focal adhesion protein and a member of the nebulin family of actin binding proteins. This recently identified splice variant of the nebulette locus is widely expressed and highly enriched in neuronal tissue. Other than that LIM-nebulette is a focal adhesion protein and interacts with zyxin, nothing is known about its function. Given that LIM-nebulette has an identical modular organization and overlapping tissue distributions to that of LASP1, we have analyzed the role of LIM-nebulette in comparison with that of LASP1. We find that LIM-nebulette is a dynamic focal adhesion protein that increases the rate of attachment and spreading of fibroblasts on fibronectin coated surfaces. Additionally, LIM-nebulette is recruited from the cortical cytoskeleton in non-motile cells to focal adhesions at the leading edge of stimulated cells. In confluent cultures of HeLa and NIH3T3 cells, LIM-nebulette co-localizes with alpha-catenin in putative adherens junctions, whereas LASP1 is devoid of these areas. Interestingly, overexpression of LIM-nebulette in PC6 cells inhibits neurite outgrowth in response to growth factors. Collectively, our data indicate that LIM-nebulette and LASP1 have distinct roles in the actin cytoskeleton.
Copyright 2008 Wiley-Liss, Inc.
Figures





References
-
- Chew CS, Chen X, Parente JA, Jr, Tarrer S, Okamoto C, Qin HY. Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci. 2002;115:4787–4799. - PubMed
-
- Chew CS, Parente JA, Jr, Chen X, Chaponnier C, Cameron RS. The LIM and SH3 domain-containing protein, lasp-1, may link the cAMP signaling pathway with dynamic membrane restructuring activities in ion transporting epithelia. J Cell Sci. 2000;113:2035–2045. - PubMed
-
- Chew CS, Parente JA, Jr, Zhou C, Baranco E, Chen X. Lasp-1 is a regulated phosphoprotein within the cAMP signaling pathway in the gastric parietal cell. Am J Physiol. 1998;275:C56–C67. - PubMed
-
- Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312:974–982. - PubMed
-
- Katoh M. Identification and characterization of LASP2 gene in silico. Int J Mol Med. 2003;12:405–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

