Auditory trace fear conditioning requires perirhinal cortex
- PMID: 18678265
- PMCID: PMC2629995
- DOI: 10.1016/j.nlm.2008.06.006
Auditory trace fear conditioning requires perirhinal cortex
Abstract
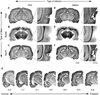
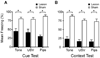
The hippocampus is well-known to be critical for trace fear conditioning, but nothing is known about the importance of perirhinal cortex (PR), which has reciprocal connections with hippocampus. PR damage severely impairs delay fear conditioning to ultrasonic vocalizations (USVs) and discontinuous tones (pips), but has no effect on delay conditioning to continuous tones. Here we demonstrate that trace auditory fear conditioning also critically depends on PR function. The trace interval between the CS offset and the US onset was 16s. Pre-training neurotoxic lesions were produced through multiple injections of N-methyl-D-aspartate along the full length of PR, which was directly visualized during the injections. Control animals received injections with phosphate-buffered saline. Three-dimensional reconstructions of the lesion volumes demonstrated that the neurotoxic damage was well-localized to PR and included most of its anterior-posterior extent. Automated video analysis quantified freezing behavior, which served as the conditional response. PR-damaged rats were profoundly impaired in trace conditioning to either of three different CSs (a USV, tone pips, and a continuous tone) as well as conditioning to the training context. Within both the lesion and control groups, the type of cue had no effect on the mean CR. The overall PR lesion effect size was 2.7 for cue conditioning and 3.9 for context conditioning. We suggest that the role of PR in trace fear conditioning may be distinct from some of its more perceptual functions. The results further define the essential circuitry underlying trace fear conditioning to auditory cues.
Figures


Similar articles
-
Perirhinal cortex supports acquired fear of auditory objects.Neurobiol Learn Mem. 2009 Jul;92(1):53-62. doi: 10.1016/j.nlm.2009.01.002. Epub 2009 Jan 29. Neurobiol Learn Mem. 2009. PMID: 19185613 Free PMC article.
-
Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals.J Neurosci. 2004 Apr 7;24(14):3610-7. doi: 10.1523/JNEUROSCI.4839-03.2004. J Neurosci. 2004. PMID: 15071109 Free PMC article.
-
Fear conditioning to discontinuous auditory cues requires perirhinal cortical function.Behav Neurosci. 2008 Oct;122(5):1178-85. doi: 10.1037/a0012902. Behav Neurosci. 2008. PMID: 18823174
-
Dual functions of perirhinal cortex in fear conditioning.Hippocampus. 2012 Oct;22(10):2068-79. doi: 10.1002/hipo.22058. Epub 2012 Aug 18. Hippocampus. 2012. PMID: 22903623 Free PMC article. Review.
-
Behavioral and neuropsychological foundations of olfactory fear conditioning.Behav Brain Res. 2000 Jun 1;110(1-2):119-28. doi: 10.1016/s0166-4328(99)00190-4. Behav Brain Res. 2000. PMID: 10802309 Review.
Cited by
-
Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning.PLoS One. 2011 Jan 19;6(1):e15982. doi: 10.1371/journal.pone.0015982. PLoS One. 2011. PMID: 21283812 Free PMC article.
-
Characterization of auditory synaptic inputs to gerbil perirhinal cortex.Front Neural Circuits. 2015 Aug 14;9:40. doi: 10.3389/fncir.2015.00040. eCollection 2015. Front Neural Circuits. 2015. PMID: 26321918 Free PMC article.
-
Modulation of intrinsic excitability as a function of learning within the fear conditioning circuit.Neurobiol Learn Mem. 2020 Jan;167:107132. doi: 10.1016/j.nlm.2019.107132. Epub 2019 Dec 9. Neurobiol Learn Mem. 2020. PMID: 31821881 Free PMC article. Review.
-
Bridging the interval: theory and neurobiology of trace conditioning.Behav Processes. 2014 Jan;101:103-11. doi: 10.1016/j.beproc.2013.08.016. Epub 2013 Sep 12. Behav Processes. 2014. PMID: 24036411 Free PMC article. Review.
-
Aging-related changes in calcium-binding proteins in rat perirhinal cortex.Neurobiol Aging. 2011 Sep;32(9):1693-706. doi: 10.1016/j.neurobiolaging.2009.10.001. Epub 2009 Nov 4. Neurobiol Aging. 2011. PMID: 19892435 Free PMC article.
References
-
- Allen TA, Brown TH. Simultaneous single-unit recordings from rat perirhinal cortex and dorsomedial prefrontal cortex during trace fear conditioning. Society for Neuroscience abstract. 2008 submitted.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

