SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes
- PMID: 18678611
- PMCID: PMC2571069
- DOI: 10.2337/dc08-0386
SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes
Abstract
Objective: The purpose of this study was to assess the glucose-lowering efficacy and safety of rimonabant monotherapy in drug-naive type 2 diabetic patients.
Research design and methods: The Study Evaluating Rimonabant Efficacy in Drug-Naive Diabetic Patients (SERENADE) was a 6-month, randomized, double-blind, placebo-controlled trial of 20 mg/day rimonabant in drug-naive patients with type 2 diabetes (A1C 7-10%). The primary end point was A1C change from baseline; secondary end points included body weight, waist circumference, and lipid profile changes.
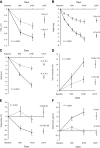
Results: A total of 281 patients were randomly assigned; 278 were exposed to treatment, and 236 (84.9%) completed the study. Baseline A1C (7.9%) was reduced by -0.8% with rimonabant versus -0.3% with placebo (Delta A1C -0.51%; P = 0.0002), with a larger rimonabant effect in patients with baseline A1C >or=8.5% (Delta A1C -1.25%; P = 0.0009). Weight loss from baseline was -6.7 kg with rimonabant versus -2.8 kg with placebo (Delta weight -3.8 kg; P < 0.0001). Rimonabant induced improvements from baseline in waist circumference (-6 vs. -2 cm; P < 0.0001), fasting plasma glucose (-0.9 vs. -0.1 mmol/l; P = 0.0012), triglycerides (-16.3 vs. +4.4%; P = 0.0031), and HDL cholesterol (+10.1 vs. +3.2%; P < 0.0001). Adverse events of interest that occurred more frequently with rimonabant versus placebo were dizziness (10.9 vs. 2.1%), nausea (8.7 vs. 3.6%), anxiety (5.8 vs. 3.6%), depressed mood (5.8 vs. 0.7%), and paresthesia (2.9 vs. 1.4%).
Conclusions: Rimonabant monotherapy resulted in meaningful improvements in glycemic control, body weight, and lipid profile in drug-naive type 2 diabetic patients. Further ongoing studies will better establish the benefit-to-risk profile of rimonabant and define its place in type 2 diabetes management.
Figures
References
-
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM: The evolving diabetes burden in the United States. Ann Intern Med 140:945–950, 2004 - PubMed
-
- King H, Aubert RE, Herman WH: Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431, 1998 - PubMed
-
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234, 1998 - PubMed
-
- Misra A, Vikram NK: Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 19:457–466, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

