Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients
- PMID: 18678616
- PMCID: PMC2570390
- DOI: 10.2337/db08-0391
Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients
Abstract
Objective: A lower in vivo mitochondrial function has been reported in both type 2 diabetic patients and first-degree relatives of type 2 diabetic patients. The nature of this reduction is unknown. Here, we tested the hypothesis that a lower intrinsic mitochondrial respiratory capacity may underlie lower in vivo mitochondrial function observed in diabetic patients.
Research design and methods: Ten overweight diabetic patients, 12 first-degree relatives, and 16 control subjects, all men, matched for age and BMI, participated in this study. Insulin sensitivity was measured with a hyperinsulinemic-euglycemic clamp. Ex vivo intrinsic mitochondrial respiratory capacity was determined in permeabilized skinned muscle fibers using high-resolution respirometry and normalized for mitochondrial content. In vivo mitochondrial function was determined by measuring phosphocreatine recovery half-time after exercise using (31)P-magnetic resonance spectroscopy.
Results: Insulin-stimulated glucose disposal was lower in diabetic patients compared with control subjects (11.2 +/- 2.8 vs. 28.9 +/- 3.7 micromol x kg(-1) fat-free mass x min(-1), respectively; P = 0.003), with intermediate values for first-degree relatives (22.1 +/- 3.4 micromol x kg(-1) fat-free mass x min(-1)). In vivo mitochondrial function was 25% lower in diabetic patients (P = 0.034) and 23% lower in first-degree relatives, but the latter did not reach statistical significance (P = 0.08). Interestingly, ADP-stimulated basal respiration was 35% lower in diabetic patients (P = 0.031), and fluoro-carbonyl cyanide phenylhydrazone-driven maximal mitochondrial respiratory capacity was 31% lower in diabetic patients (P = 0.05) compared with control subjects with intermediate values for first-degree relatives.
Conclusions: A reduced basal ADP-stimulated and maximal mitochondrial respiratory capacity underlies the reduction in in vivo mitochondrial function, independent of mitochondrial content. A reduced capacity at both the level of the electron transport chain and phosphorylation system underlies this impaired mitochondrial capacity.
Figures

 , FDR; ▪, type 2 diabetic patients. *P < 0.05 compared with diabetic patients.
, FDR; ▪, type 2 diabetic patients. *P < 0.05 compared with diabetic patients.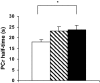
 , FDR; ▪, type 2 diabetic patients. *P < 0.05 compared with diabetic patients.
, FDR; ▪, type 2 diabetic patients. *P < 0.05 compared with diabetic patients.
 , FDR; ▪, type 2 diabetic patients. *P < 0.05 compared with diabetic patients.
, FDR; ▪, type 2 diabetic patients. *P < 0.05 compared with diabetic patients.References
-
- Kelley DE, He J, Menshikova EV, Ritov VB: Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 - PubMed
-
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100: 8466–8471, 2003 - PMC - PubMed
-
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC: PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

