Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3
- PMID: 18678617
- PMCID: PMC2570388
- DOI: 10.2337/db08-0079
Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3
Abstract
Objective: Fatty acid-induced mitochondrial uncoupling and oxidative stress have been proposed to reduce cardiac efficiency and contribute to cardiac dysfunction in type 2 diabetes. We hypothesized that mitochondrial uncoupling may also contribute to reduced cardiac efficiency and contractile dysfunction in the type 1 diabetic Akita mouse model (Akita).
Research design and methods: Cardiac function and substrate utilization were determined in isolated working hearts and in vivo function by echocardiography. Mitochondrial function and coupling were determined in saponin-permeabilized fibers, and proton leak kinetics was determined in isolated mitochondria. Hydrogen peroxide production and aconitase activity were measured in isolated mitochondria, and total reactive oxygen species (ROS) were measured in heart homogenates.
Results: Resting cardiac function was normal in Akita mice, and myocardial insulin sensitivity was preserved. Although Akita hearts oxidized more fatty acids, myocardial O(2) consumption was not increased, and cardiac efficiency was not reduced. ADP-stimulated mitochondrial oxygen consumption and ATP synthesis were decreased, and mitochondria showed grossly abnormal morphology in Akita. There was no evidence of oxidative stress, and despite a twofold increase in uncoupling protein 3 (UCP3) content, ATP-to-O ratios and proton leak kinetics were unchanged, even after perfusion of Akita hearts with 1 mmol/l palmitate.
Conclusions: Insulin-deficient Akita hearts do not exhibit fatty acid-induced mitochondrial uncoupling, indicating important differences in the basis for mitochondrial dysfunction between insulin-responsive type 1 versus insulin-resistant type 2 diabetic hearts. Increased UCP3 levels do not automatically increase mitochondrial uncoupling in the heart, which supports the hypothesis that fatty acid-induced mitochondrial uncoupling as exists in type 2 diabetic hearts requires a concomitant increase in ROS generation.
Figures

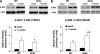




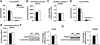
References
-
- Boudina S, Abel ED: Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology 21: 250–258, 2006 - PubMed
-
- Bugger H, Abel ED: Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci 114: 195–210, 2008 - PubMed
-
- Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED: Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53: 2366–2374, 2004 - PubMed
-
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED: Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349, 2005 - PubMed
-
- Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED: Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112: 2686–2695, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

