Sprouty2-mediated inhibition of fibroblast growth factor signaling is modulated by the protein kinase DYRK1A
- PMID: 18678649
- PMCID: PMC2547015
- DOI: 10.1128/MCB.00394-08
Sprouty2-mediated inhibition of fibroblast growth factor signaling is modulated by the protein kinase DYRK1A
Abstract
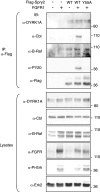
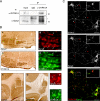
Raf-MEK-extracellular signal-regulated kinase (Erk) signaling initiated by growth factor-engaged receptor tyrosine kinases (RTKs) is modulated by an intricate network of positive and negative feedback loops which determine the specificity and spatiotemporal characteristics of the intracellular signal. Well-known antagonists of RTK signaling are the Sprouty proteins. The activity of Sprouty proteins is modulated by phosphorylation. However, little is known about the kinases responsible for these posttranslational modifications. We identify DYRK1A as one of the protein kinases of Sprouty2. We show that DYRK1A interacts with and regulates the phosphorylation status of Sprouty2. Moreover, we identify Thr75 on Sprouty2 as a DYRK1A phosphorylation site in vitro and in vivo. This site is functional, since its mutation enhanced the repressive function of Sprouty2 on fibroblast growth factor (FGF)-induced Erk signaling. Further supporting the idea of a functional interaction, DYRK1A and Sprouty2 are present in protein complexes in mouse brain, where their expression overlaps in several structures. Moreover, both proteins copurify with the synaptic plasma membrane fraction of a crude synaptosomal preparation and colocalize in growth cones, pointing to a role in nerve terminals. Our results suggest, therefore, that DYRK1A positively regulates FGF-mitogen-activated protein kinase signaling by phosphorylation-dependent impairment of the inhibitory activity of Sprouty2.
Figures






References
-
- Alvarez, M., X. Estivill, and S. de la Luna. 2003. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J. Cell Sci. 1163099-3107. - PubMed
-
- Arron, J. R., M. M. Winslow, A. Polleri, C. P. Chang, H. Wu, X. Gao, J. R. Neilson, L. Chen, J. J. Heit, S. K. Kim, N. Yamasaki, T. Miyakawa, U. Francke, I. A. Graef, and G. R. Crabtree. 2006. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 441595-600. - PubMed
-
- Benavides-Piccione, R., M. Dierssen, I. Ballesteros-Yanez, M. Martinez de Lagran, M. L. Arbones, V. Fotaki, J. DeFelipe, and G. N. Elston. 2005. Alterations in the phenotype of neocortical pyramidal cells in the Dyrk1A+/− mouse. Neurobiol. Dis. 20115-122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
