Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes
- PMID: 18678651
- PMCID: PMC2547012
- DOI: 10.1128/MCB.00021-08
Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes
Abstract
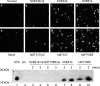
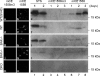
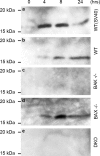
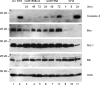
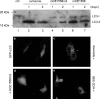
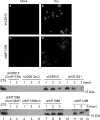
Addition of a 5' cap to RNA polymerase II transcripts, the first step of pre-mRNA processing in eukaryotes from yeasts to mammals, is catalyzed by the sequential action of RNA triphosphatase, guanylyltransferase, and (guanine-N-7)methyltransferase. The effects of knockdown of these capping enzymes in mammalian cells were investigated using T7 RNA polymerase-synthesized small interfering RNA and also a lentivirus-based inducible, short hairpin RNA system. Decreasing either guanylyltransferase or methyltransferase resulted in caspase-3 activation and elevated terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining characteristic of apoptosis. Induction of apoptosis was independent of p53 tumor suppressor but dependent on BAK or BAX. In addition, levels of the BH3 family member Bim increased, while Mcl-1 and Bik levels remained unchanged during apoptosis. In contrast to capping enzyme knockdown, apoptosis induced by cycloheximide inhibition of protein synthesis required BAK but not BAX. Both Bim and Mcl-1 levels decreased in cycloheximide-induced apoptosis while Bik levels were unchanged, suggesting that apoptosis in siRNA-treated cells is not a direct consequence of loss of mRNA translation. siRNA-treated BAK(-/-) BAX(-/-) double-knockout mouse embryonic fibroblasts failed to activate capase-3 or increase TUNEL staining but instead exhibited autophagy, as demonstrated by proteolytic processing of microtubule-associated protein 1 light chain 3 (LC3) and translocation of transfected green fluorescent protein-LC3 from the nucleus to punctate cytoplasmic structures.
Figures



References
-
- Asanuma, K., I. Tanida, I. Shirato, T. Ueno, H. Takahara, T. Nishitani, E. Kominami, and Y. Tomino. 2003. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 171165-1167. - PubMed
-
- Boatright, K. M., and G. S. Salvesen. 2003. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15725-731. - PubMed
-
- Caelles, C., A. Helmberg, and M. Karin. 1994. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370220-223. - PubMed
-
- Danial, N. N. 2007. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin. Cancer Res. 137254-7263. - PubMed
-
- Fernandes-Alnemri, T., R. C. Armstrong, J. Krebs, S. M. Srinivasula, L. Wang, F. Bullrich, L. C. Fritz, J. A. Trapani, K. J. Tomaselli, G. Litwack, and E. S. Alnemri. 1996. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc. Natl. Acad. Sci. USA 937464-7469. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
