Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis
- PMID: 18678708
- PMCID: PMC2500131
- DOI: 10.1083/jcb.200802113
Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis
Abstract
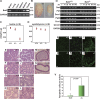
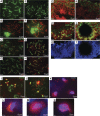
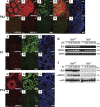
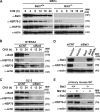
Meiosis is critical for sexual reproduction. During meiosis, the dynamics and integrity of homologous chromosomes are tightly regulated. The genetic and molecular mechanisms governing these processes in vivo, however, remain largely unknown. In this study, we demonstrate that Bat3/Scythe is essential for survival and maintenance of male germ cells (GCs). Targeted inactivation of Bat3/Scythe in mice results in widespread apoptosis of meiotic male GCs and complete male infertility. Pachytene spermatocytes exhibit abnormal assembly and disassembly of synaptonemal complexes as demonstrated by abnormal SYCP3 staining and sustained gamma-H2AX and Rad51/replication protein A foci. Further investigation revealed that a testis-specific protein, Hsp70-2/HspA2, is absent in Bat3-deficient male GCs at any stage of spermatogenesis; however, Hsp70-2 transcripts are expressed at normal levels. We found that Bat3 deficiency induces polyubiquitylation and subsequent degradation of Hsp70-2. Inhibition of proteasomal degradation restores Hsp70-2 protein levels. Our findings identify Bat3 as a critical regulator of Hsp70-2 in spermatogenesis, thereby providing a possible molecular target in idiopathic male infertility.
Figures

References
-
- Allen, J.W., D.J. Dix, B.W. Collins, B.A. Merrick, C. He, J.K. Selkirk, P. Poorman-Allen, M.E. Dresser, and E.M. Eddy. 1996. HSP70-2 is part of the synaptonemal complex in mouse and hamster spermatocytes. Chromosoma. 104:414–421. - PubMed
-
- Anway, M.D., J. Folmer, W.W. Wright, and B.R. Zirkin. 2003. Isolation of sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function. Biol. Reprod. 68:996–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

