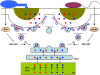
Secretory pathways in plant immune responses
- PMID: 18678749
- PMCID: PMC2492620
- DOI: 10.1104/pp.108.121566
Secretory pathways in plant immune responses
Figures
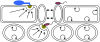

Similar articles
-
Transport and secretion in plant-microbe interactions.Curr Opin Plant Biol. 2007 Dec;10(6):573-9. doi: 10.1016/j.pbi.2007.08.002. Epub 2007 Sep 17. Curr Opin Plant Biol. 2007. PMID: 17875397 Review.
-
Recognition of pathogens by plants.Curr Biol. 2000 Jan 13;10(1):R5-7. doi: 10.1016/s0960-9822(99)00273-0. Curr Biol. 2000. PMID: 10660284 Review. No abstract available.
-
Plant science. Auxin begins to give up its secrets.Science. 2006 Sep 1;313(5791):1230-1. doi: 10.1126/science.313.5791.1230. Science. 2006. PMID: 16946049 No abstract available.
-
Cooperative ethylene receptor signaling.Plant Signal Behav. 2012 Aug;7(8):1009-13. doi: 10.4161/psb.20937. Epub 2012 Jul 25. Plant Signal Behav. 2012. PMID: 22827938 Free PMC article. Review.
-
Q&A: How do plants respond to ethylene and what is its importance?BMC Biol. 2016 Jan 27;14:7. doi: 10.1186/s12915-016-0230-0. BMC Biol. 2016. PMID: 26819080 Free PMC article.
Cited by
-
High-throughput confocal imaging of intact live tissue enables quantification of membrane trafficking in Arabidopsis.Plant Physiol. 2010 Nov;154(3):1096-104. doi: 10.1104/pp.110.160325. Epub 2010 Sep 14. Plant Physiol. 2010. PMID: 20841454 Free PMC article.
-
A highway for war and peace: the secretory pathway in plant-microbe interactions.Mol Plant. 2011 Jul;4(4):581-7. doi: 10.1093/mp/ssr053. Epub 2011 Jul 7. Mol Plant. 2011. PMID: 21742620 Free PMC article. Review.
-
A Simple Method for Measuring Apoplast Hydration and Collecting Apoplast Contents.Plant Physiol. 2019 Apr;179(4):1265-1272. doi: 10.1104/pp.18.01076. Epub 2019 Mar 1. Plant Physiol. 2019. PMID: 30824565 Free PMC article.
-
Time-resolved dual transcriptomics reveal early induced Nicotiana benthamiana root genes and conserved infection-promoting Phytophthora palmivora effectors.BMC Biol. 2017 May 11;15(1):39. doi: 10.1186/s12915-017-0379-1. BMC Biol. 2017. PMID: 28494759 Free PMC article.
-
Induction of DREB2A pathway with repression of E2F, jasmonic acid biosynthetic and photosynthesis pathways in cold acclimation-specific freeze-resistant wheat crown.Funct Integr Genomics. 2013 Mar;13(1):57-65. doi: 10.1007/s10142-012-0303-2. Epub 2012 Dec 20. Funct Integr Genomics. 2013. PMID: 23262780
References
-
- Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14 145–163
-
- Bais HP, Prithiviraj B, Jha AK, Ausubel FM, Vivanco JM (2005) Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature 434 217–221 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

