Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197
- PMID: 18678836
- PMCID: PMC2654376
- DOI: 10.1200/JCO.2008.16.7841
Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197
Abstract
Purpose: The combination of doxorubicin and cyclophosphamide (AC) is a standard adjuvant regimen. Doxorubicin and docetaxel (AT) is one of the most active cytotoxic regimens for metastatic breast cancer. The purpose of this trial was to determine whether adjuvant AT improved disease-free survival compared with AC in operable breast cancer.
Patients and methods: Women with invasive breast cancer were eligible if there were one to three positive lymph nodes or if the node-negative tumor was greater than 1 cm. Patients were randomly assigned after surgery to receive doxorubicin (60 mg/m(2)) plus either cyclophosphamide (600 mg/m(2); AC) or docetaxel (60 mg/m(2); AT) given every 3 weeks for four cycles, followed by hormone therapy for patients with estrogen receptor (ER) and/or progesterone receptor (PR)-positive tumors.
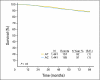
Results: There were 2,882 eligible patients enrolled. After a median follow-up of 79.5 months, there was no significant difference in disease-free survival (DFS; 85% in both arms) or overall survival (91% v 92%) at 5 years. The hazard ratio for AC versus AT was 1.02 (95% CI for DFS, 0.86 to 1.22; P = .78). In an exploratory analysis of prespecified stratification factors by ER and PR expression there were trends toward improved DFS for AT in ER/PR-negative disease. Grade 3 neutropenia associated with fever or infection occurred more often with AT (26% v 10%; P < .05).
Conclusion: AT did not improve DFS or overall survival in this population, and was associated with more toxicity.
Figures




References
-
- Fisher B, Brown AM, Dimitrov NV, et al: Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8:1483-1496, 1990 - PubMed
-
- Bishop JF, Dewar J, Toner GC, et al: Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol 17:2355-2364, 1999 - PubMed
-
- Chan S, Friedrichs K, Noel D, et al: Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17:2341-2354, 1999 - PubMed
-
- Crown J, Dieras V, Kaufmann M, et al: Chemotherapy for metastatic breast cancer-report of a European expert panel. Lancet Oncol 3:719-727, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

