Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial
- PMID: 18678843
- PMCID: PMC3201984
- DOI: 10.7326/0003-4819-149-3-200808050-00005
Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial
Abstract
Background: Women with urge urinary incontinence are commonly treated with antimuscarinic medications, but many discontinue therapy.
Objective: To determine whether combining antimuscarinic drug therapy with supervised behavioral training, compared with drug therapy alone, improves the ability of women with urge incontinence to achieve clinically important reductions in incontinence episodes and to sustain these improvements after discontinuing drug therapy.
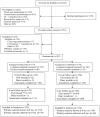
Design: 2-stage, multicenter, randomized clinical trial conducted from July 2004 to January 2006.
Setting: 9 university-affiliated outpatient clinics.
Patients: 307 women with urge-predominant incontinence.
Intervention: 10 weeks of open-label, extended-release tolterodine alone (n = 153) or combined with behavioral training (n = 154), followed by discontinuation of therapy and follow-up at 8 months.
Measurements: The primary outcome, measured at 8 months, was no receipt of drugs or other therapy for urge incontinence and a 70% or greater reduction in frequency of incontinence episodes. Secondary outcomes were reduction in incontinence, self-reported satisfaction and improvement, and scores on validated questionnaires measuring symptom distress and bother and health-related quality of life. Study staff who performed outcome evaluations, but not participants and interventionists, were blinded to group assignment.
Results: 237 participants completed the trial. According to life-table estimates, the rate of successful discontinuation of therapy at 8 months was the same in the combination therapy and drug therapy alone groups (41% in both groups; difference, 0 percentage points [95% CI, -12 to 12 percentage points]). A higher proportion of participants who received combination therapy than drug therapy alone achieved a 70% or greater reduction in incontinence at 10 weeks (69% vs. 58%; difference, 11 percentage points [CI, -0.3 to 22.1 percentage points]). Combination therapy yielded better outcomes over time on the Urogenital Distress Inventory and the Overactive Bladder Questionnaire (both P <0.001) at both time points for patient satisfaction and perceived improvement but not health-related quality of life. Adverse events were uncommon (12 events in 6 participants [3 in each group]).
Limitations: Behavioral therapy components (daily bladder diary and recommendations for fluid management) in the group receiving drug therapy alone may have attenuated between-group differences. Assigned treatment was completed by 68% of participants, whereas 8-month outcome status was assessed on 77%.
Conclusion: The addition of behavioral training to drug therapy may reduce incontinence frequency during active treatment but does not improve the ability to discontinue drug therapy and maintain improvement in urinary incontinence. Combination therapy has a beneficial effect on patient satisfaction, perceived improvement, and reduction of other bladder symptoms.
Trial registration: ClinicalTrials.gov NCT00090584.
Figures

References
-
- Fantl JA, Newman DK, Colling J, et al. Clinical Practice Guideline, No 2. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research; 1996. Urinary Incontinence in Adults: Acute and Chronic Management. Update. AHCPR Publication No. 96-0682.
-
- Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology. 1998;51:355–61. - PubMed
-
- Coyne KS, Zhou Z, Thompson C, Versi E. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92:731–735. - PubMed
-
- Seim A, Eriksen BC, Hunskaar S. A study of female urinary incontinence in general practice. Demography, medical history, and clinical findings. Scand J Urol Nephrol. 1996;30:465–471. - PubMed
-
- Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 DK60393/DK/NIDDK NIH HHS/United States
- U01 DK058234/DK/NIDDK NIH HHS/United States
- U01 DK58234/DK/NIDDK NIH HHS/United States
- U01 DK060393/DK/NIDDK NIH HHS/United States
- U01 DK60379/DK/NIDDK NIH HHS/United States
- U01 DK060380/DK/NIDDK NIH HHS/United States
- U01 DK060397/DK/NIDDK NIH HHS/United States
- U01 DK60380/DK/NIDDK NIH HHS/United States
- U01 DK60395/DK/NIDDK NIH HHS/United States
- U01 DK060401/DK/NIDDK NIH HHS/United States
- U01 DK060395/DK/NIDDK NIH HHS/United States
- U01 DK58229/DK/NIDDK NIH HHS/United States
- U01 DK60397/DK/NIDDK NIH HHS/United States
- U01 DK58231/DK/NIDDK NIH HHS/United States
- U01 DK060379/DK/NIDDK NIH HHS/United States
- U01 DK60401/DK/NIDDK NIH HHS/United States
- U01 DK058229/DK/NIDDK NIH HHS/United States
- U01 DK58225/DK/NIDDK NIH HHS/United States
- U01 DK058225/DK/NIDDK NIH HHS/United States
- U01 DK058231/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
