Identification of Mg2+ -dependent neutral sphingomyelinase 1 as a mediator of heat stress-induced ceramide generation and apoptosis
- PMID: 18678863
- PMCID: PMC2662064
- DOI: 10.1074/jbc.M805402200
Identification of Mg2+ -dependent neutral sphingomyelinase 1 as a mediator of heat stress-induced ceramide generation and apoptosis
Abstract
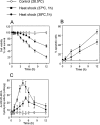
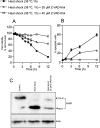
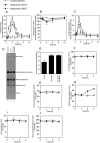
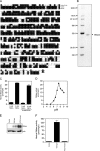
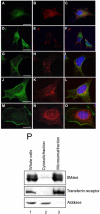
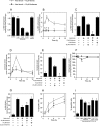
Neutral sphingomyelinases (SMases) are involved in the induction of ceramide-mediated proapoptotic signaling under heat stress conditions. Although ceramide is an important mediator of apoptosis, the neutral SMase that is activated under heat stress has not been identified. In this study, we cloned an Mg(2+)-dependent neutral SMase from a zebrafish embryonic cell cDNA library using an Escherichia coli expression-cloning vector. Screening of the clones using an SMase activity assay with C(6)-7-nitro-2-1,3-benzoxadiazol-4-yl-sphingomyelin as the substrate resulted in the isolation of one neutral SMase cDNA clone. This cDNA encoded a polypeptide of 420 amino acids (putative molecular weight: 46,900) containing two predicted transmembrane domains in its C-terminal region. The cloned neutral SMase 1 acted as a mediator of stress-induced apoptosis. Bacterially expressed recombinant neutral SMase 1 hydrolyzed [choline-methyl-(14)C]sphingomyelin optimally at pH 7.5 in the presence of an Mg(2+) ion. In zebrafish embryonic cells, the endogenous SMase enzyme was localized in the microsomal fraction. In FLAG-tagged SMase-overexpressing cells, neutral SMase 1 colocalized with a Golgi marker in a cytochemical analysis. Inactivation of the enzyme by an antisense phosphorothioate oligonucleotide repressed the induction of ceramide generation, caspase-3 activation, and apoptotic cell death by heat stress. Thus, neutral SMase 1 participates in an inducible ceramide-mediating, proapoptotic signaling pathway that operates in heat-induced apoptosis in zebrafish embryonic cells.
Figures
Similar articles
-
A novel mitochondrial sphingomyelinase in zebrafish cells.J Biol Chem. 2009 Jul 24;284(30):20349-63. doi: 10.1074/jbc.M109.004580. Epub 2009 May 8. J Biol Chem. 2009. PMID: 19429680 Free PMC article.
-
Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3.J Biol Chem. 1999 Apr 9;274(15):10654-60. doi: 10.1074/jbc.274.15.10654. J Biol Chem. 1999. PMID: 10187863
-
Molecular cloning, characterization, and expression of a novel human neutral sphingomyelinase.J Biol Chem. 1999 Dec 24;274(52):37407-12. doi: 10.1074/jbc.274.52.37407. J Biol Chem. 1999. PMID: 10601312
-
Ceramide and sphingomyelinases in the regulation of stress responses.Chem Phys Lipids. 1999 Nov;102(1-2):141-7. doi: 10.1016/s0009-3084(99)00082-1. Chem Phys Lipids. 1999. PMID: 11001568 Review.
-
Neutral sphingomyelinase: past, present and future.Chem Phys Lipids. 1999 Nov;102(1-2):79-96. doi: 10.1016/s0009-3084(99)00077-8. Chem Phys Lipids. 1999. PMID: 11001563 Review.
Cited by
-
The dynamics and role of sphingolipids in eukaryotic organisms upon thermal adaptation.Prog Lipid Res. 2020 Nov;80:101063. doi: 10.1016/j.plipres.2020.101063. Epub 2020 Sep 2. Prog Lipid Res. 2020. PMID: 32888959 Free PMC article. Review.
-
Unbalanced Sphingolipid Metabolism and Its Implications for the Pathogenesis of Psoriasis.Molecules. 2020 Mar 3;25(5):1130. doi: 10.3390/molecules25051130. Molecules. 2020. PMID: 32138315 Free PMC article. Review.
-
Ceramide in stress response.Adv Exp Med Biol. 2010;688:86-108. doi: 10.1007/978-1-4419-6741-1_6. Adv Exp Med Biol. 2010. PMID: 20919648 Free PMC article. Review.
-
The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes.J Biol Chem. 2015 Feb 6;290(6):3455-67. doi: 10.1074/jbc.M114.605253. Epub 2014 Dec 10. J Biol Chem. 2015. PMID: 25505180 Free PMC article.
-
The essential neutral sphingomyelinase is involved in the trafficking of the variant surface glycoprotein in the bloodstream form of Trypanosoma brucei.Mol Microbiol. 2010 Jun;76(6):1461-82. doi: 10.1111/j.1365-2958.2010.07151.x. Epub 2010 Apr 1. Mol Microbiol. 2010. PMID: 20398210 Free PMC article.
References
-
- Obeid, L. M., Linardic, C. M., Karolak, L. A., and Hannun, Y. A. (1993) Science 259 1769-1771 - PubMed
-
- Pena, L. A., Fuks, Z., and Kolesnick, R. (1997) Biochem. Pharmacol. 53 615-621 - PubMed
-
- Okazaki, T., Bell, R. M., and Hannun, Y. A. (1989) J. Biol. Chem. 264 19076-19080 - PubMed
-
- Hannun, Y. A., and Luberto, C. (2000) Trends Cell Biol. 10 73-80 - PubMed
-
- Obeid, L. M., and Hannun, Y. A. (2003) Sci. Aging Knowledge Environ. 2003 27 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

