Proteinase-activated receptor 2 stimulates Na,K-ATPase and sodium reabsorption in native kidney epithelium
- PMID: 18678869
- PMCID: PMC2661380
- DOI: 10.1074/jbc.M804399200
Proteinase-activated receptor 2 stimulates Na,K-ATPase and sodium reabsorption in native kidney epithelium
Abstract
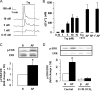
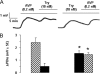
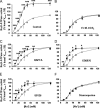
Proteinase-activated receptors 2 (PAR2) are expressed in kidney, but their function is mostly unknown. Since PAR2 control ion transport in several epithelia, we searched for an effect on sodium transport in the cortical thick ascending limb of Henle's loop, a nephron segment that avidly reabsorbs NaCl, and for its signaling. Activation of PAR2, by either trypsin or a specific agonist peptide, increased the maximal activity of Na,K-ATPase, its apparent affinity for sodium, the sodium permeability of the paracellular pathway, and the lumen-positive transepithelial voltage, featuring increased NaCl reabsorption. PAR2 activation induced calcium signaling and phosphorylation of ERK1,2. PAR2-induced stimulation of Na,K-ATPase Vmax was fully prevented by inhibition of phospholipase C, of changes in intracellular concentration of calcium, of classical protein kinases C, and of ERK1,2 phosphorylation. PAR2-induced increase in paracellular sodium permeability was mediated by the same signaling cascade. In contrast, increase in the apparent affinity of Na,K-ATPase for sodium, although dependent on phospholipase C, was independent of calcium signaling, was insensitive to inhibitors of classical protein kinases C and of ERK1,2 phosphorylation, but was fully prevented by the nonspecific protein kinase inhibitor staurosporine, as was the increase in transepithelial voltage. In conclusion, PAR2 increases sodium reabsorption in rat thick ascending limb of Henle's loop along both the transcellular and the paracellular pathway. PAR2 effects are mediated in part by a phospholipase C/protein kinase C/ERK1,2 cascade, which increases Na,K-ATPase maximal activity and the paracellular sodium permeability, and by a different phospholipase C-dependent, staurosporine-sensitive cascade that controls the sodium affinity of Na,K-ATPase.
Figures




Similar articles
-
C-peptide stimulates Na+,K+-ATPase activity via PKC alpha in rat medullary thick ascending limb.Diabetologia. 2003 Jan;46(1):124-31. doi: 10.1007/s00125-002-0996-1. Epub 2003 Jan 9. Diabetologia. 2003. PMID: 12637991
-
Effect of cAMP on the activity and the phosphorylation of Na+,K(+)-ATPase in rat thick ascending limb of Henle.Kidney Int. 1999 May;55(5):1819-31. doi: 10.1046/j.1523-1755.1999.00414.x. Kidney Int. 1999. PMID: 10231444
-
Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control.Physiol Rev. 2001 Jan;81(1):345-418. doi: 10.1152/physrev.2001.81.1.345. Physiol Rev. 2001. PMID: 11152761 Review.
-
ERK1/2 controls Na,K-ATPase activity and transepithelial sodium transport in the principal cell of the cortical collecting duct of the mouse kidney.J Biol Chem. 2004 Dec 3;279(49):51002-12. doi: 10.1074/jbc.M405674200. Epub 2004 Sep 28. J Biol Chem. 2004. PMID: 15456767
-
AVP dynamically increases paracellular Na+ permeability and transcellular NaCl transport in the medullary thick ascending limb of Henle's loop.Pflugers Arch. 2017 Jan;469(1):149-158. doi: 10.1007/s00424-016-1915-5. Epub 2016 Dec 6. Pflugers Arch. 2017. PMID: 27924355 Review.
Cited by
-
Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition.Am J Physiol Cell Physiol. 2009 Aug;297(2):C459-70. doi: 10.1152/ajpcell.00201.2009. Epub 2009 Jun 17. Am J Physiol Cell Physiol. 2009. PMID: 19535514 Free PMC article.
-
Differential localization patterns of claudin 10, 16, and 19 in human, mouse, and rat renal tubular epithelia.Am J Physiol Renal Physiol. 2021 Aug 1;321(2):F207-F224. doi: 10.1152/ajprenal.00579.2020. Epub 2021 Jun 21. Am J Physiol Renal Physiol. 2021. PMID: 34151590 Free PMC article.
-
Renal proteinase-activated receptor 2, a new actor in the control of blood pressure and plasma potassium level.J Biol Chem. 2013 Apr 5;288(14):10124-10131. doi: 10.1074/jbc.M112.446393. Epub 2013 Feb 19. J Biol Chem. 2013. PMID: 23430254 Free PMC article.
-
A new methodology for quantification of alternatively spliced exons reveals a highly tissue-specific expression pattern of WNK1 isoforms.PLoS One. 2012;7(5):e37751. doi: 10.1371/journal.pone.0037751. Epub 2012 May 31. PLoS One. 2012. PMID: 22701532 Free PMC article.
-
WNK1-related Familial Hyperkalemic Hypertension results from an increased expression of L-WNK1 specifically in the distal nephron.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14366-71. doi: 10.1073/pnas.1304230110. Epub 2013 Aug 12. Proc Natl Acad Sci U S A. 2013. PMID: 23940364 Free PMC article.
References
-
- Ossovskaya, V. S., and Bunnett, N. W. (2004) Physiol. Rev. 84 579–621 - PubMed
-
- Fyfe, M., Bergstrom, M., Aspengren, S., and Peterson, A. (2005) Cytokine 31 358–367 - PubMed
-
- Kawao, N., Nagataki, M., Nagasawa, K., Kubo, S., Cushing, K., Wada, T., Sekiguchi, F., Ichida, S., Hollenberg, M. D., MacNaughton, W. K., Nishikawa, H., and Kawabata, A. (2005) J. Pharmacol. Exp. Ther. 315 576–589 - PubMed
-
- Nishibori, M., Mori, S., and Takahashi, H. K. (2005) J. Pharmacol. Sci. 97 25–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

