Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease
- PMID: 18678882
- PMCID: PMC2583907
- DOI: 10.1369/jhc.2008.951855
Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease
Abstract
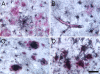
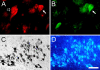
The proinflammatory enzyme 5-lipoxygenase (5-LOX) is upregulated in Alzheimer's disease (AD), but its localization and association with the hallmark lesions of the disease, beta-amyloid (Abeta) plaques and neurofibrillary tangles (NFTs), is unknown. This study examined the distribution and cellular localization of 5-LOX in the medial temporal lobe from AD and control subjects. The spatial relationship between 5-LOX immunoreactive structures and AD lesions was also examined. We report that, in AD subjects, 5-LOX immunoreactivity is elevated relative to controls, and its localization is dependent on the antibody-targeted portion of the 5-LOX amino acid sequence. Carboxy terminus-directed antibodies detected 5-LOX in glial cells and neurons, but less frequently in neurons with dystrophic (NFT) morphology. In contrast, immunoreactivity observed using 5-LOX amino terminus-directed antibodies was virtually absent in neurons and abundant in NFTs, neuritic plaques, and glia. Double-labeling studies showed a close association of 5-LOX-immunoreactive processes and glial cells with Abeta immunoreactive plaques and vasculature and also detected 5-LOX in tau immunoreactive and amyloid containing NFTs. Different immunolabeling patterns with antibodies against carboxy vs amino terminus of 5-LOX may be caused by post-translational modifications of 5-LOX protein in Abeta plaques and NFTs. The relationship between elevated intracellular 5-LOX and hallmark AD pathological lesions provides further evidence that neuroinflammatory pathways contribute to the pathogenesis of AD.
Figures



Similar articles
-
Immunohistochemical analysis of hippocampal butyrylcholinesterase: Implications for regional vulnerability in Alzheimer's disease.Neuropathology. 2016 Apr;36(2):135-45. doi: 10.1111/neup.12241. Epub 2015 Aug 21. Neuropathology. 2016. PMID: 26293308 Free PMC article.
-
Activation of caspase-6 in aging and mild cognitive impairment.Am J Pathol. 2007 Apr;170(4):1200-9. doi: 10.2353/ajpath.2007.060974. Am J Pathol. 2007. PMID: 17392160 Free PMC article.
-
Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain.Neurobiol Dis. 2002 Nov;11(2):341-54. doi: 10.1006/nbdi.2002.0549. Neurobiol Dis. 2002. PMID: 12505426
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Viewpoint: Crosstalks between neurofibrillary tangles and amyloid plaque formation.Ageing Res Rev. 2013 Jan;12(1):174-81. doi: 10.1016/j.arr.2012.06.002. Epub 2012 Jun 19. Ageing Res Rev. 2013. PMID: 22728532 Review.
Cited by
-
The 5-lipoxygenase pathway: oxidative and inflammatory contributions to the Alzheimer's disease phenotype.Front Cell Neurosci. 2015 Jan 14;8:436. doi: 10.3389/fncel.2014.00436. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25642165 Free PMC article. Review.
-
Specialized Pro-resolving Lipid Mediators and Glial Cells: Emerging Candidates for Brain Homeostasis and Repair.Front Cell Neurosci. 2021 Apr 26;15:673549. doi: 10.3389/fncel.2021.673549. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33981203 Free PMC article. Review.
-
The Role of Eicosanoids in Alzheimer's Disease.Int J Environ Res Public Health. 2019 Jul 18;16(14):2560. doi: 10.3390/ijerph16142560. Int J Environ Res Public Health. 2019. PMID: 31323750 Free PMC article. Review.
-
Can inflammation be resolved in Alzheimer's disease?Ther Adv Neurol Disord. 2018 Aug 9;11:1756286418791107. doi: 10.1177/1756286418791107. eCollection 2018. Ther Adv Neurol Disord. 2018. PMID: 30116300 Free PMC article. Review.
-
Lipoxygenases and poly(ADP-ribose) polymerase in amyloid beta cytotoxicity.Neurochem Res. 2011 May;36(5):839-48. doi: 10.1007/s11064-011-0412-7. Epub 2011 Feb 2. Neurochem Res. 2011. PMID: 21287270 Free PMC article.
References
-
- Bishnoi M, Patil CS, Kumar A, Kulkarni SK (2005) Protective effects of nimesulide (COX inhibitor), AKBA (5-LOX inhibitor), and their combination in aging-associated abnormalities in mice. Methods Find Exp Clin Pharmacol 27:465–470 - PubMed
-
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259 - PubMed
-
- Chinnici CM, Yao Y, Praticò D (2007) The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging 28:1457–1462 - PubMed
-
- Cuzzocrea S, Rossi A, Mazzon E, Di Paola R, Genovese T, Muià C, Caputi AP, et al. (2005) 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab Invest 85:808–822 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

