Demyelination arrest and remyelination induced by glatiramer acetate treatment of experimental autoimmune encephalomyelitis
- PMID: 18678887
- PMCID: PMC2516229
- DOI: 10.1073/pnas.0804632105
Demyelination arrest and remyelination induced by glatiramer acetate treatment of experimental autoimmune encephalomyelitis
Abstract
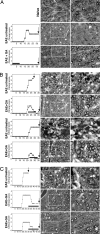
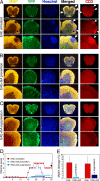
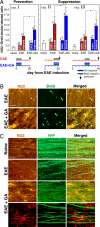
The interplay between demyelination and remyelination is critical in the progress of multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). In the present study, we explored the capacity of glatiramer acetate (GA, Copaxone) to affect the demyelination process and/or lead to remyelination in mice inflicted by chronic EAE, using both scanning electron microscopy and immunohistological methods. Spinal cords of untreated EAE mice revealed substantial demyelination accompanied by tissue destruction and axonal loss. In contrast, in spinal cords of GA-treated mice, in which treatment started concomitantly with disease induction (prevention), no pathology was observed. Moreover, when treatment was initiated after the appearance of clinical symptoms (suppression) or even in the chronic disease phase (delayed suppression) when substantial demyelination was already manifested, it resulted in a significant decrease in the pathological damage. Detection of oligodendrocyte progenitor cells (OPCs) expressing the NG2 or O4 markers via colocalization with the proliferation marker BrdU indicated their elevated levels in spinal cords of GA-treated mice. The mode of action of GA in this system is attributed to increased proliferation, differentiation, and survival of OPCs along the oligodendroglial maturation cascade and their recruitment into injury sites, thus enhancing repair processes in situ.
Conflict of interest statement
Conflict of interest statement: Michael Sela and Ruth Arnon are among the inventors of Copaxone.
Figures

References
-
- Lassmann H. In: McAlpin's Multiple Sclerosis. Compston A, et al., editors. Oxford: Churchill Livingstone; 1998. pp. 323–358.
-
- Lassmann H. Experimental models of multiple sclerosis. Rev Neurol. 2007;163:651–655. - PubMed
-
- Baumann N, Phan-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–926. - PubMed
-
- Bruck W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. - PubMed
-
- Reynolds R, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

