Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach
- PMID: 18678895
- PMCID: PMC2516212
- DOI: 10.1073/pnas.0800069105
Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach
Erratum in
- Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20558
Abstract
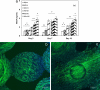
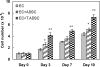
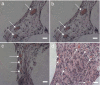
One of the fundamental principles underlying tissue engineering approaches is that newly formed tissue must maintain sufficient vascularization to support its growth. Efforts to induce vascular growth into tissue-engineered scaffolds have recently been dedicated to developing novel strategies to deliver specific biological factors that direct the recruitment of endothelial cell (EC) progenitors and their differentiation. The challenge, however, lies in orchestration of the cells, appropriate biological factors, and optimal factor doses. This study reports an approach as a step forward to resolving this dilemma by combining an ex vivo gene transfer strategy and EC transplantation. The utility of this approach was evaluated by using 3D poly(lactide-co-glycolide) (PLAGA) sintered microsphere scaffolds for bone tissue engineering applications. Our goal was achieved by isolation and transfection of adipose-derived stromal cells (ADSCs) with adenovirus encoding the cDNA of VEGF. We demonstrated that the combination of VEGF releasing ADSCs and ECs results in marked vascular growth within PLAGA scaffolds. We thereby delineate the potential of ADSCs to promote vascular growth into biomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
VEGF-incorporated biomimetic poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering.J Biomed Mater Res B Appl Biomater. 2012 Nov;100(8):2187-96. doi: 10.1002/jbm.b.32787. Epub 2012 Aug 22. J Biomed Mater Res B Appl Biomater. 2012. PMID: 22915492
-
Tissue engineering of bladder using vascular endothelial growth factor gene-modified endothelial progenitor cells.Int J Artif Organs. 2011 Dec;34(12):1137-46. doi: 10.5301/ijao.5000069. Int J Artif Organs. 2011. PMID: 22198599
-
Engineering vascularized soft tissue flaps in an animal model using human adipose-derived stem cells and VEGF+PLGA/PEG microspheres on a collagen-chitosan scaffold with a flow-through vascular pedicle.Biomaterials. 2015 Dec;73:198-213. doi: 10.1016/j.biomaterials.2015.09.024. Epub 2015 Sep 18. Biomaterials. 2015. PMID: 26410787 Free PMC article.
-
Osteogenesis and angiogenesis: the potential for engineering bone.Eur Cell Mater. 2008 May 2;15:100-14. doi: 10.22203/ecm.v015a08. Eur Cell Mater. 2008. PMID: 18454418 Review.
-
Adipose-Derived Stem Cells: Angiogenetic Potential and Utility in Tissue Engineering.Int J Mol Sci. 2024 Feb 16;25(4):2356. doi: 10.3390/ijms25042356. Int J Mol Sci. 2024. PMID: 38397032 Free PMC article. Review.
Cited by
-
Pretreatment Glasgow Prognostic Score Correlated with Serum Histidine Level and Three-Year Mortality of Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma and Optimal Performance Status.Nutrients. 2022 Aug 24;14(17):3475. doi: 10.3390/nu14173475. Nutrients. 2022. PMID: 36079741 Free PMC article.
-
Comprehensive genetic analysis of early host body reactions to the bioactive and bio-inert porous scaffolds.PLoS One. 2014 Jan 14;9(1):e85132. doi: 10.1371/journal.pone.0085132. eCollection 2014. PLoS One. 2014. PMID: 24454803 Free PMC article.
-
Polymer-ceramic spiral structured scaffolds for bone tissue engineering: effect of hydroxyapatite composition on human fetal osteoblasts.PLoS One. 2014 Jan 27;9(1):e85871. doi: 10.1371/journal.pone.0085871. eCollection 2014. PLoS One. 2014. PMID: 24475056 Free PMC article.
-
Dipeptide-based polyphosphazene and polyester blends for bone tissue engineering.Biomaterials. 2010 Jun;31(18):4898-908. doi: 10.1016/j.biomaterials.2010.02.058. Epub 2010 Mar 23. Biomaterials. 2010. PMID: 20334909 Free PMC article.
-
Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing.Mol Ther. 2013 Feb;21(2):445-55. doi: 10.1038/mt.2012.234. Epub 2012 Nov 20. Mol Ther. 2013. PMID: 23164936 Free PMC article.
References
-
- Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA. Tissue engineering: Orthopedic applications. Annu Rev Biomed Eng. 1990;1:19–46. - PubMed
-
- Burg KJL, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–2359. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell- delivery systems. J Biomater Sci Polymer Edn. 2004;15:701–726. - PubMed
-
- Sharkawy AA, Kiltzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40:598–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

