The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex
- PMID: 18678899
- PMCID: PMC2495013
- DOI: 10.1073/pnas.0804918105
The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex
Abstract
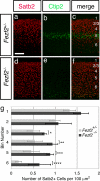
Pyramidal neurons in the deep layers of the cerebral cortex can be classified into two major classes: callosal projection neurons and long-range subcortical neurons. We and others have shown that a gene expressed specifically by subcortical projection neurons, Fezf2, is required for the formation of axonal projections to the spinal cord, tectum, and pons. Here, we report that Fezf2 regulates a decision between subcortical vs. callosal projection neuron fates. Fezf2(-/-) neurons adopt the fate of callosal projection neurons as assessed by their axonal projections, electrophysiological properties, and acquisition of Satb2 expression. Ctip2 is a major downstream effector of Fezf2 in regulating the extension of axons toward subcortical targets and can rescue the axonal phenotype of Fezf2 mutants. When ectopically expressed, either Fezf2 or Ctip2 can alter the axonal targeting of corticocortical projection neurons and cause them to project to subcortical targets, although Fezf2 can promote a subcortical projection neuron fate in the absence of Ctip2 expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- McConnell SK. Constructing the cerebral cortex: Neurogenesis and fate determination. Neuron. 1995;15:761–768. - PubMed
-
- O'Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. - PubMed
-
- Miller MW. In: Cerebral Cortex: Development and Maturation of Cerebral Cortex. Peter A, Jones EG, editors. Vol 7. New York: Plenum; 1998. pp. 133–175.
-
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: A review. Brain Res. 1973;62:1–35. - PubMed
-
- Luskin MB, Shatz CJ. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985;242:611–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

