An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor
- PMID: 18678906
- PMCID: PMC2504787
- DOI: 10.1073/pnas.0801845105
An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor
Abstract
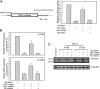
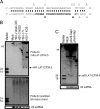
Latency-associated transcript (LAT) sequences regulate herpes simplex virus (HSV) latency and reactivation from sensory neurons. We found a HSV-2 LAT-related microRNA (miRNA) designated miR-I in transfected and infected cells in vitro and in acutely and latently infected ganglia of guinea pigs in vivo. miR-I is also expressed in human sacral dorsal root ganglia latently infected with HSV-2. miR-I is expressed under the LAT promoter in vivo in infected sensory ganglia. We also predicted and identified a HSV-1 LAT exon-2 viral miRNA in a location similar to miR-I, implying a conserved mechanism in these closely related viruses. In transfected and infected cells, miR-I reduces expression of ICP34.5, a key viral neurovirulence factor. We hypothesize that miR-I may modulate the outcome of viral infection in the peripheral nervous system by functioning as a molecular switch for ICP34.5 expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
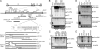

Similar articles
-
Herpes simplex virus 2 microRNA miR-H6 is a novel latency-associated transcript-associated microRNA, but reduction of its expression does not influence the establishment of viral latency or the recurrence phenotype.J Virol. 2011 May;85(9):4501-9. doi: 10.1128/JVI.01997-10. Epub 2011 Feb 16. J Virol. 2011. PMID: 21325410 Free PMC article.
-
Novel less-abundant viral microRNAs encoded by herpes simplex virus 2 latency-associated transcript and their roles in regulating ICP34.5 and ICP0 mRNAs.J Virol. 2009 Feb;83(3):1433-42. doi: 10.1128/JVI.01723-08. Epub 2008 Nov 19. J Virol. 2009. PMID: 19019961 Free PMC article.
-
Characterization of herpes simplex virus 2 primary microRNA Transcript regulation.J Virol. 2015 May;89(9):4837-48. doi: 10.1128/JVI.03135-14. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673716 Free PMC article.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
The latency-associated gene of herpes simplex virus type 1 (HSV-1) interferes with superinfection by HSV-1.J Neurovirol. 2002 Dec;8 Suppl 2:97-102. doi: 10.1080/13550280290167920. J Neurovirol. 2002. PMID: 12491159 Review.
Cited by
-
A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM.J Virol. 2012 Dec;86(23):12891-902. doi: 10.1128/JVI.01055-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993162 Free PMC article.
-
Noncoding RNPs of viral origin.Cold Spring Harb Perspect Biol. 2011 Mar 1;3(3):a005165. doi: 10.1101/cshperspect.a005165. Cold Spring Harb Perspect Biol. 2011. PMID: 20719877 Free PMC article. Review.
-
Immunomodulatory roles of human herpesvirus-encoded microRNA in host-virus interaction.Rev Med Virol. 2020 Jan;30(1):e2081. doi: 10.1002/rmv.2081. Epub 2019 Aug 20. Rev Med Virol. 2020. PMID: 31432608 Free PMC article. Review.
-
HSV-2-encoded miRNA-H4 Regulates Cell Cycle Progression and Act-D-induced Apoptosis in HeLa Cells by Targeting CDKL2 and CDKN2A.Virol Sin. 2019 Jun;34(3):278-286. doi: 10.1007/s12250-019-00101-8. Epub 2019 Apr 5. Virol Sin. 2019. PMID: 30953292 Free PMC article.
-
Hidden regulation of herpes simplex virus 1 pre-mRNA splicing and polyadenylation by virally encoded immediate early gene ICP27.PLoS Pathog. 2019 Jun 17;15(6):e1007884. doi: 10.1371/journal.ppat.1007884. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31206552 Free PMC article.
References
-
- Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

