Crystal structure of the Agrobacterium virulence complex VirE1-VirE2 reveals a flexible protein that can accommodate different partners
- PMID: 18678909
- PMCID: PMC2516231
- DOI: 10.1073/pnas.0801525105
Crystal structure of the Agrobacterium virulence complex VirE1-VirE2 reveals a flexible protein that can accommodate different partners
Abstract
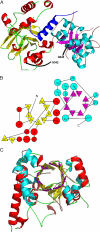
Agrobacterium tumefaciens infects its plant hosts by a mechanism of horizontal gene transfer. This capability has led to its widespread use in artificial genetic transformation. In addition to DNA, the bacterium delivers an abundant ssDNA binding protein, VirE2, whose roles in the host include protection from cytoplasmic nucleases and adaptation for nuclear import. In Agrobacterium, VirE2 is bound to its acidic chaperone VirE1. When expressed in vitro in the absence of VirE1, VirE2 is prone to oligomerization and forms disordered filamentous aggregates. These filaments adopt an ordered solenoidal form in the presence of ssDNA, which was characterized previously by electron microscopy and three-dimensional image processing. VirE2 coexpressed in vitro with VirE1 forms a soluble heterodimer. VirE1 thus prevents VirE2 oligomerization and competes with its binding to ssDNA. We present here a crystal structure of VirE2 in complex with VirE1, showing that VirE2 is composed of two independent domains presenting a novel fold, joined by a flexible linker. Electrostatic interactions with VirE1 cement the two domains of VirE2 into a locked form. Comparison with the electron microscopy structure indicates that the VirE2 domains adopt different relative orientations. We suggest that the flexible linker between the domains enables VirE2 to accommodate its different binding partners.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chilton MD, et al. Stable incorporation of plasmid DNA into higher plant cells: The molecular basis of crown gall tumorigenesis. Cell. 1977;11:263–271. - PubMed
-
- Zupan J, Muth TR, Draper O, Zambryski P. The transfer of DNA from agrobacterium tumefaciens into plants: A feast of fundamental insights. Plant J. 2000;23:11–28. - PubMed
-
- Tzfira T, Citovsky V. Agrobacterium-mediated genetic transformation of plants: Biology and biotechnology. Curr Opin Biotechnol. 2006;17:147–154. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

